Many people still believe that cold fusion is the result of bad science. In contrast, numerous laboratories in at least 10 countries have now claimed production of anomalous energy using a variety of methods, many of which are now reproducible. This energy is proposed to result from nuclear reactions initiated within a special periodic array of atoms at modest temperatures (energy). Evidence for nuclear reactions involving fusion of deuterium, transmutation involving both light and heavy hydrogen, and nuclear interaction between heavy nuclei has been published. The claims, if true, reveal a new method to release nuclear energy without harmful radiation and without the radioactivity associated with conventional methods. This paper examines published evidence describing this new phenomenon in order to test its reality and to extend an understanding of the process.
1. INTRODUCTION
In 1989, Pons and Fleischmann [1] (P-F) caused a media storm by claiming to cause fusion to take place in an ordinary electrolytic cell containing D2O. This process was first named "Cold Fusion" by Steven Jones - an especially poor description. The names "Chemically Assisted Nuclear Reactions" (CANR) and "Low Energy Nuclear Reactions" (LENR) more correctly describe the phenomenon. Over 21 papers by P-F describe their work in more detail, a few of the more important ones are noted here. [2-5] Their claim was soon rejected because it could not be easily replicated and it could not be explained using information available at the time. From six months after the announcement until now, the subject has been ignored by the scientific community and is considered by many to be pathological science. In spite of this general rejection, work continues, initially in ten countries and now in six countries, with official government support in most cases. These studies have provided a large number of observations and have answered most of the questions posed by skeptics. Even though the results defy conventional explanation, it is time for this information to be known by the general scientific community, regardless of its interpretation. If true, the observations reveal a completely new method to initiate nuclear reactions within an atomic structure. On the other hand, if these claims are false, as many people believe, a large number of highly trained scientists, using well understood equipment, can not be trusted to obtain accurate data, thereby calling into question conclusions reached in other fields based on the same techniques.
The phenomenon is claimed to produce fusion as well as a complex mixture of transmutation reactions. Twelve different methods, listed in Table 1, have been reported to produce anomalous energy (AE) and/or nuclear products (NP), some with good reproducibility and some with difficulty. Most of these methods produce many of the same effects using both hydrogen and deuterium. Consequently, light water can not be used as a null (blank) test of the method as some people have suggested. Naturally, theoreticians have been busy trying to structure these observations into a model. Over 500 models have been suggested with perhaps a dozen giving useful insight. A few of these ideas will be discussed later in the paper. Regardless of the explanation du jour, more energy appears to be produced than is being applied, thereby violating basic thermodynamic expectations. The important question is, "Why"?
|
|
|
The literature of the field now contains over 3000 publications on the subject with about 1000 being important to an understanding, only a fraction of which are discussed here. Unfortunately, much of this information is not available in "normal" scientific journals to which a reader has easy access. When possible, easily available, peer reviewed journals will be cited even though this will result in much useful information being ignored. A few of the references in this paper are to conference proceedings because the data are available only from this source. Listings of available references and the hard-to-find publications are available to serious students and can be obtained from several sources. [6-9]
This paper has three major objectives: to give a general overview of the field so that interested scientists can understand the individual observations, to answer some of the major challenges raised by skeptics, and to suggest several new ideas for future study. The method used by Pons and Fleischmann will be the primary focus of the discussion because this is the most thoroughly studied method. A few other methods are introduced briefly to show that the effect can be produced in several different ways to give similar patterns of behavior. Hopefully, this effort will reawaken interest in this fascinating subject.
2. DISCUSSION
Several basic questions must be answered before the claims can be believed.
1. Have the claims been replicated?
2. Can prosaic errors or processes explain the effect?
3. Are reproducible patterns of behavior observed?
4. Can the effect be produced using various methods?
5. Why has the effect been so hard to replicate?
6. Does the anomalous energy have a nuclear source?
7. Can these claims be explained?
These questions were asked in 1989 and only now, 12 years later, can suitable answers be provided. While the answers given here may not satisfy everyone, hopefully they will lessen doubt about the phenomenon being real and encourage more people to study the effect.
QUESTION #1. HAS ANOMALOUS ENERGY BEEN REPLICATED?
As noted in Table 1, many of the methods have been successfully replicated. Because the P-F method has been given the greatest attention, this method will be used to answer Question #1. A few other methods will be examined briefly as an answer to Question #4. Please note that some of these methods use little or no applied power to produce the AE.
First, the nature of the so-called anomalous energy produced by the P-F method needs to be understood. Claimed anomalous energy (AE) is detected as heat and measured using a calorimeter. Anomalous power (AP) is defined as the difference between the amount of heat (watts) being removed from the calorimeter, based on a previous calibration, and the amount being applied as electrical power, which is calculated as voltage across the cell times current through the cell. Anomalous energy is the product of anomalous power and time. Table 2 shows a listing of AP using the P-F method. These results were obtained by different laboratories, at different times, using different calorimeters. Notice that for these examples, the amount of AP is always well above the claimed uncertainty. In addition, many of these studies report finding numerous active samples even though only the most active one is listed in the table. Many other studies either failed to detect AP or the results were marginal. The wide range of values might indicate systematic error, but it also is consistent with a variable amount of active material being involved in the process. Do these facts have any meaning other than that everyone is making the same mistake? An answer must come from an understanding of how the studies were done, as discussed in Questions #2 and #3.
|
(reference details) |
|
|
OPEN(a) |
Watts |
MAXIMUM AP(b) |
|
Huang et al. [1] |
|
|
|
|
2.3, 450 |
|
Kainthla et al. [2] |
|
|
|
|
1.08, 468 |
|
Samthanam et al. [3] |
|
|
|
|
1.54, 63 |
|
Appleby et al. [4] |
|
|
|
|
0.0457, 600 |
|
Beizner et al. [5] |
|
|
|
|
~1, ~500 |
|
Eagleton and Bush [6] |
|
|
|
|
6.0, 450 |
|
Scott et al. [7] |
|
|
|
|
2.0, 600 |
|
Fleischmann et al. [8] |
|
|
|
|
2.8, 1024 |
|
Hutchinson[9] |
|
|
|
|
4.0, 250 |
|
Zahn[10] |
|
|
|
|
~2, 124 |
|
Miles et al. [11] |
|
|
|
|
0.3, 100 |
|
Oriani et al. [12] |
|
|
|
|
3.2, >1000 |
|
Yang et al, [13] |
|
|
|
|
9.1, ? |
|
Zhang[14] |
|
|
|
|
0.15, ~15 |
|
Bertalot et al.[15] |
|
|
|
|
0.08, 650 |
|
Bush et al. [16] |
|
|
|
|
0.52, 227 |
|
McKubre et al. [17] |
|
|
|
|
0.5, 660 |
|
Noninski[18] |
|
|
|
|
2.6, 80 |
|
Yun et al. [19] |
|
|
Open and closed |
|
0.24, 500 |
|
Bertalot et al. [20] |
|
|
|
|
3.0, 190 |
|
Gozzi et al. [21] |
|
|
|
|
9.0, ? |
|
Hasegawa et al. [22] |
|
|
|
|
~0.7, ? |
|
McKubre et al. [23] |
|
|
|
|
1.2, 440 |
|
Ota et al. [24] |
|
|
|
|
1.0, ? |
|
Storms[25] |
|
|
|
|
7.5, 700 |
|
Okamoto et al. [26] |
|
|
|
|
6.0, 66 |
|
Storms[27] |
|
|
|
|
2.0, 600 |
|
Bertalot et al. [28] |
|
|
|
|
11, 2000 |
|
Takahashi et al. [29] |
|
|
|
|
3.5, ? |
|
Kamimura et al. [30] |
|
|
|
|
0.700, 800 |
|
Yasuda et al. [31] |
|
|
|
|
5.0, ? |
|
Ota, et al. [32] |
|
|
|
|
0.29, 750 |
|
Szpak et al. [33] |
|
|
|
|
0.4, 133 |
|
Storms [34] |
|
|
|
|
0.8, 0.75 |
(a)
(b) Although only one value is given, frequently several different samples of palladium were reported to produce anomalous power (AP). The amount of anomalous energy (AE) is highly variable, depending on how long the active sample was studied.
(c) Calibration could have been unstable.
(d) Calibrated with internal heater and checked with Pt cathode and/or H2O based electrolyte
(e) Calibrated only with internal heater
(f) Mechanical stirring used
(g) Calibrated using only an inert cathode.
QUESTION #2: CAN PROSAIC ERROR OR PROCESSES EXPLAIN THE EFFECT?
An answer requires all possible errors be identified, something that is not always possible to do. The major errors will be evaluated first.
All calorimeters need to be calibrated and various methods are used to do this. A Joule (resistive) heater can be placed within the cell and a known amount of electrical energy applied. A better method is to apply electrolytic power while using an inert cathode. This method is sometimes possible while using a palladium cathode because bulk palladium frequently takes many days before AE is detected. This delay provides an opportunity to calibrate the calorimeter by applying various amounts of electrolytic power to the cell before AP starts. The calibration method used is very important when evaluating potential errors. Several of the listed studies used more than one method.
Each calorimeter design has its own set of potential errors. These errors can be evaluated in several different ways provided sufficient information is given in the publication, a condition that is seldom met. One method uses statistics by assuming all errors are random. However, most error is not random, but instead results from overlooked deficiencies within the apparatus producing a bias or off-set. The difference being that random error produces an error band in which any change in energy is hidden, while an off-set results in apparent energy that may be anomalous or may be caused by error in the measurement resulting from a prosaic process. A few such problems associated with the electrolytic method have been identified [10-16]. Especially important are uncertain recombination[17] of D2 and O2 gases being generated within the cell, unrecognized temperature gradients [18, 19], and a variable thermal conductivity of the cell wall [20], each of which is described below in more detail.
Many errors are completely avoided by using a flow-type, Seebeck-type, or the double-wall isoperibolic-type calorimeter containing a recombination catalyst. These improved techniques do not suffer from errors attributed to early studies. Rather than causing the anomalous effects to disappear, these improved methods are found to produce the same behavior as obtained using less sophisticated calorimetry, as can be seen in Table 2. Consequently, the effects have not gone away when better equipment is used, a condition that must be met before the popular concept of pathological science can be applied. [21]
Because P-F started the field, their work has been subjected to especially sharp analysis [11, 14, 22-31]. Their study is no longer the only source of support for their claims because the effect has been duplicated many times using much better equipment, as described in a later section. Nevertheless, the most recent evaluation concludes that their claims for AE are justified[32] based solely on their work.
The following prosaic explanations for the claimed AE have been suggested.
Release of hydrogen gas[33]
Deuterium gas, as it is released by the palladium, is proposed to carry away heat, thereby cooling the cathode and depositing this energy in the electrolyte where it might be mistaken for excess energy. In addition, energy is then proposed to leak into the cell through the cooled cathode lead from the warmer outside.
Because all electrolytic cells are thermodynamically closed systems, energy used to compress the hydrogen (deuterium) within the cathode will exactly equal energy produced by its release. No net energy will be generated under this condition. In any case, an inert cathode would exhibit the same effect during calibration, thereby canceling the effect, should it be real.
Production of hydrogen gas [34]
Release of atomic deuterium from the Pd lattice is proposed to produce bursts of heat as it combines to form D2 gas and as it reacts with any surface oxide.
This explanation is not possible because under steady-state conditions deuterium enters the palladium as fast as it leaves. At most, this effect could only explain small bursts of energy, not steady production normally reported. Also, no surface oxide remains on a Pd surface once electrolysis starts.
Recombination [17, 35]
The importance of recombination was recognized by everyone in the field since 1989. Although recombining catalysts were not used at first, care was taken to measure the amount of D2O leaving the cell as D2 and O2 gas. Most studies, including P-F, found this effect to be less than 1 percent. In addition, subsequent studies have shown that recombination is only a problem when a very low applied current (below 100 mA) is used.[32, 36-38] In any case, most studies reporting AE now use an internal catalyst to convert all D2 and O2 back to D2O, thereby completely eliminating this source of potential error.
Release of stored stress [39-42]
Most samples of Pd have been annealed before their use, hence contain very little stress. Stress is introduced into palladium when it reacts with hydrogen (deuterium). While this stress might be released suddenly by crack formation, thereby producing a burst of energy, such release can not account for the observed large, continuous production of AE under steady-state conditions. Some studies have reported an amount of AE greater than an equivalent weight of TNT, an energy well outside of any possible energy storage mechanism.
Current fluctuations [43]
Bubble action is proposed to cause high frequency fluctuations in both current and voltage. Because DC measurements are made, these AC components could be missed by the data acquisition system. As a result, the amount of power applied to the cell would be too small compared to the amount being measured by the calorimeter, hence excess power would be proposed.
Such spikes on the applied DC voltage have been measured using a high frequency scope while AP was being produced and found to be less than 50 mV.[44] In addition, the same missing power would occur when the cell is calibrated using a dead cathode or the Pd cathode before AP is generated. As a result, the effect would cancel out. Also, people who have calibrated using both an internal heater, which does not produce this AC component, and a dead cathode report excellent agreement between the resulting calibration constants, thereby suggesting this error is not present, as Holst-Hansen and Britz concluded [43].
Peltier Effect [45]
A difference in Peltier coefficients between the metals used as cathode and anode is proposed to cause pumping of energy into the cell by applied current.
Two problems exist in this model. The proposed mathematical formula leads to an unrealistic conclusion, i.e. that an infinite amount of heat can be introduced into the cell when the temperature difference between interior and exterior approaches zero. The other problem is that the effect, if real, only becomes important when unrealistic differences in Peltier coefficients are proposed. In any case, use of electrolytic calibration would subtract such heat from the final result, thereby canceling any effect.
Chemical reactions [46]
Excess heat is proposed to result from the formation of chemical compounds within the electrolyte caused by the electrolytic current.
The chemicals used in a typical electrolytic cell are all stable and do not interact chemically unless a current is applied. This current can initiate various chemical reactions including reaction of D2 with the cathode to form PdD, reaction of O2 with the Pt anode to form PtO, reaction of Li with the cathode to form an alloy, and the rare formation of D2O2. These reactions are very slow, involve very little energy, and are endothermic. Such a chemical product would have to be more stable than the most stable compound known to exist in order to account for the observed energy. No chemical product even close to this requirement has ever been found within an active cell.
Temperature gradients [14]
Temperature gradients are proposed to exist within the electrolyte, which causes the recorded temperature to be unstable. As a result, EP based on this temperature would be unstable and potentially wrong.
Any cell design that uses the interior temperature to determine the loss-rate of heat is susceptible to this problem. Normally, bubble action reduces this gradient to insignificant values during electrolysis.[20, 47, 48] However, calibration using an internal heater can lead to unexpected error because no bubble action would be present to reduce the temperature gradient. Therefore, all studies using only this method of calibration are suspect unless electrolytic current is applied during heater calibration, as was done in some studies including those of P-F.
Variable thermal conductivity of the wall [20]
A stagnant layer of fluid exists next to the cell wall and this layer affects the apparent thermal conductivity of the wall. Because a single-wall isoperibolic calorimeter is sensitive to the thermal conductivity of the wall, changes in this stagnant layer, caused by mechanical stirring or convection currents produced by bubble action, change the calibration constant. Fortunately, the calorimeter used by P-F lost most of its energy by radiation through the wall and by conduction through the lid. Therefore, their values were not affected by this problem. Some other studies have not been so fortunate.
Jahn-Teller effect [49]
Energy is proposed to be generated by D atoms dissolving in PdD, combining to form D2 molecules within a new structure, and then leaving as D2 gas. To quote the author, "excess heat of cold fusion appears to be nothing more than the storage and release of the latent vaporization heat of heavy water".
This process ignores the fact that the same amount of energy would be used to place D within PdD as would be released when the D2 leaves. In a closed system, under steady state conditions, energy can not be generated unless a permanent change takes place in either the chemical or physical structure. For this explanation to apply, D2O in the electrolyte would have to change its properties by losing energy. After sufficient time, this energy would be exhausted and the remaining D2O would have much different properties compared to fresh D2O. No evidence for this change has ever been seen. If this energy were stored during initial loading of the palladium cathode, the process would be seen as an endothermic reaction having a value equal to the amount of energy released later. No such storage and release process has been seen in any study.
Errors in Calibration Constants [50]
The calibration constant of a flow calorimeter, or any type of calorimeter for that matter, is proposed to fluctuate such that periodic EP can be observed when a fixed value for the calibration constant is used. This variation can take two forms. If a calorimeter is stable, the constant will show random fluctuations around a mean, generated by minor variations in the measurement of temperature and applied power. When a calorimeter is unstable, the constant will show a steady drift with time. The assertion made here is that the actual sensitivity of a calorimeter will fluctuate even though the calorimeter appears to be stable, thereby generating periodic bursts of apparent EP.
This assertion has been applied to only one unique study [51] in which EP was found to result when the applied current was increased in steps over a range and then decreased in the same manner. Presumably, changes in the calibration constant occurred at the exact moment when each of the seven such current scans was started and this change always resulted in similar behavior, i.e. a rising and then a falling EP. While such coincidence can not be totally ruled out, the proposed error can not be applied to the many studies that report EP lasting for hours and days, with periodic calibrations being made during this time.
Although the processes described above apply only to the P-F method, no prosaic process has provided a satisfactory explanation for all positive results using the other methods (Table 1), especially when AE and NP are produced in the absence of applied power. This does not mean such an explanation is not possible. It only means that the probability of finding a prosaic explanation has been significantly reduced. To make the challenge still more difficult, AE has now been detected under a wide range of conditions using many different calorimeters, all of which gave the same basic behavior. This effectively rules out a single prosaic process. We are now left with a situation such that several errors must occur, each giving the same apparent basic behavior regardless of the apparatus or method being used. This is a very weak basis for believing error is the only cause of the observations.
QUESTION 3: ARE REPRODUCIBLE PATTERNS OF BEHAVIOR OBSERVED?
Presence of reproducible patterns within the data sets are as important as achieving reproducibility of the phenomenon its self. Such patterns are based on AE being produced only under certain unique conditions by all studies. For example, a pattern can be claimed if AE is only detected when the D/Pd ratio is above a critical value, or if nuclear products are always found after AE production, regardless of the method used. Production of nuclear products will be discussed in Question #6.
Lack of space prevents each method listed in Table 1 from being described in detail. Instead, only a few especially compelling investigations will be examined. First, the P-F method will be described below, followed by a few other methods as answers to Question #4.
The work at SRI, lead by Dr. McKubre, studied the phenomena, first with $6M provided by EPRI (Electric Power Research Institute). After this program was terminated, work was continued by IMRA (Japan) at nearly the same level. A very small effort is now being funded by the US government. Over the course of this work, several designs of flow-type calorimeters were used that share the following characteristics:
1. The cells are sealed and contain a recombiner. As a result, no gas leaves the cell. Therefore, uncertainty in the amount of recombination is not an issue. Successful action by the recombiner is monitored using different methods including change in gas pressure.
2. The cells contain a heater, which maintains a constant inner temperature. Power to this heater can be adjusted to compensate for any change in temperature caused by electrolysis or by anomalous processes. This heater is also used to determine whether the power measurement, based on the flow rate and temperature change of a cooling fluid, is accurate. Sensitivity better than ±0.01 W (±0.1%) is claimed.
3. The electrolytic cell, its surrounding heater, and the cooling-fluid channels are all contained within a silvered, evacuated Dewar in order to isolate them from the environment.
4. The whole assembly is immersed in a fluid bath, which maintains a constant environment of 30±0.003°C. This bath is also the source of cooling fluid. Consequently, most studies are done at a temperature above 30°C.
5. A constant flow pump is used to circulate cooling fluid. Flow rate is checked periodically by weighing the fluid passing through the calorimeter. Better than 98% of power produced within the cell is captured in this fluid.
6. All aspects of the measurement are under computer control, which provides continuous monitoring, and redundant RTD sensors are used for temperature measurement.
7. The deuterium content of the palladium cathode is determined by measuring its change in resistance.

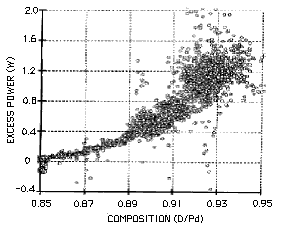
FIGURE 1. Relationship between average composition of PdD and the amount of anomalous (excess) power produced in a Pons-Fleischmann cell. This study was done at SRI.
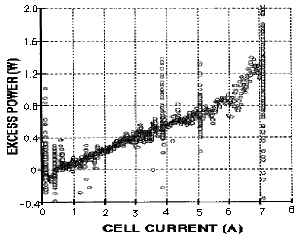
FIGURE 2. Relationship between anomalous (excess) power and current applied to a Pons-Fleischmann cell containing a Pd cathode and a D2O+LiOD electrolyte.The study was done at SRI.
Nineteen samples of palladium were found to make AP with consistent results. One consistent behavior is shown in Figure 1 as the relationship between AP and average composition of the cathode. Figure 2 shows another pattern as the effect of applied current on AP. These two behaviors are found to be produced by all samples of palladium used in this work and by all samples reported in the literature when the necessary measurements were made. Variations in reported values are easily explained by the different shapes and sizes of the cathodes used, and by the amount of active material present on their surfaces. In addition, the following behaviors are also seen by everyone who has made suitable measurements.
1. The average D/Pd ratio must exceed a critical value. This value differs somewhat between studies because only the average composition can be determined and the value depends on the method used and the shape of the cathode. Typically, the value lies between D/Pd=0.85 and 0.90. Infrequently, compositions above this range are found to be dead for unknown reasons.
2. The current must be maintained for a critical time. This time is variable and presumably depends on how rapidly the surface can acquire the active structure. The time is zero for thin layers of Pd while it can be as long as months for bulk palladium. Failure to wait the necessary time is one reason some people have not seen the effect.
3. The current density must be above a critical value. Applied current determines the surface composition, hence the nature of the active structure. A value above 150 mA/cm2 is usually found for bulk palladium. No critical value appears to be necessary for thin layers of palladium.
4. Inert palladium can sometimes be activated by addition of certain impurities to the electrolyte. These impurities are found to help the surface achieve a higher deuterium content.
5. The effect occurs in only a small fraction of samples, but more often in certain batches than in other ones. In fact, all physical properties of palladium are found to be batch specific, making this metal highly variable in its general behavior, even in conventional applications.
These patterns of behavior add evidence that the observations are a real behavior of nature and not caused by error.
QUESTION #4: CAN THE EFFECT BE PRODUCED USING VARIOUS METHODS?
Eight other techniques have also produce AE besides electrolysis, two of which are described below. Of course, some attempts to replicate these methods have failed. Even successful studies have many failures before success is achieved. As argued below with respect to the P-F method, failure only gives an insight when the reasons are known, not when the reasons are based on speculation. Many of the reasons for failure using the P-F method are now known and will be discussed in Question #5.
Deuterium gas applied to finely divided palladium:
Prof. Arata {Note: Prof. Arata is the only physicist to be awarded the Emperor's Prize in Japan for his contribution to the Japanese hot fusion program.} and Dr. Zhang (Japan) [52-55] developed a way to load finely divided palladium powder (palladium-black) with very pure deuterium and measured the resulting AE and NP. The apparatus consists of a sealed palladium tube containing powdered palladium. This is used as the cathode in an electrolytic cell containing D2O. Deuterium is generated at the cathode surface, diffuses through the palladium wall, and accumulates inside where it reacts with the palladium-black. Consequently, electrolytic action is only used to generate very pure D2 gas, in contrast to the P-F method. These scientists find that after the D2 pressure had increased to several atmospheres, AP is generated. Anomalous energy production can be maintained for months and it is accompanied by helium and tritium generation. This work has been replicated at SRI [56] using a flow-type calorimeter different from the one used by Arata and Zhang. The relationship between the AP and applied power is shown in Fig. 3, where the AE produced using H2O and D2O is compared. Clarke et al. [57] failed to find He-4 in a sample of the Pd-black used in the SRI study, but did detect He-3 that resulted from the decay of tritium [58]. Based on the decay rate, this tritium apparently was produced during the SRI study.
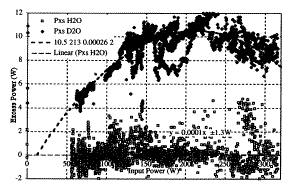
FIGURE 3. Relationship between applied power and anomalous power produced by an Arata-type cell containing palladium-black using an electrolyte containing D2O or H2O. This study was done at SRI with the help of Prof. Arata.
Dr. Case[59, 60] explored a variation of this method by using a commercial catalyst consisting of finely divided palladium deposited on charcoal. This material has the advantage that the isolated Pd particles will not lose their small size by sintering together when the material is heated, unlike particles in palladium-black which are in contact. When this material is heated to about 250° C with a temperature gradient and about 3 atm of D2 is applied, AP is produced along with helium. This work has been replicated at least 5 times at SRI.[56] The relationship between AP, based on two independent methods, and helium measured in the gas is shown in Fig. 4. Helium concentration in the gas eventually exceeds 5.2 ppm, the measured concentration in the laboratory air. Consequently, this helium can not result from leaks in the system. Also, the initial catalyst was tested for absorbed helium and found to be essentially free of this element. The implied energy for the helium producing reaction shown in the figure is an upper limit because some helium is retained by the catalyst. When this helium is measured and taken into account, the value is consistent with the expected value of 23.8 MeV/helium.
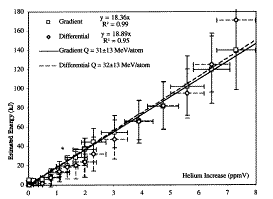
FIGURE 4. Relationship between the amount of anomalous energy and the amount of helium produced in a cell containing Pd on charcoal surrounded by D2 gas. This study was done at SRI with the help of Drs. Case and George.
These two methods show the same patterns of behavior, i.e. finely divided Pd produces AE and NP when exposed to D2, but not when exposed to H2. As noted below, finely divided Pd also is found to make AE when it is applied to an inert cathode surface in a P-F cell. [61]
It is important to realize that success using these two gas-loading methods depends critically on being able to remove impurities from the surface of the small palladium particles, not an easy task. Also, the presence of light hydrogen is expected to be a poison for the nuclear reaction, similar to its behavior in a P-F cell.
Proton conductor:
A number of semiconductors can dissolve a little hydrogen (deuterium) and become conducting by electromigration of hydrogen ions when the material is heated and a voltage is applied. Because current is very small, the amount of applied power is much less than used for a P-F cell. Use of deuterium is reported to result in AE and occasional transmutation. Ordinary hydrogen shows no effect.
Mizuno et al.[62, 63] made discs using a mixture of strontium, cerium, yttrium and niobium oxides, with platinum applied to opposite sides as electrical contacts. Upon heating to 400-700°C and introducing D2 at 0.1-50 Torr, five out of fifty discs were found to make AE. Gamma emission was observed and attributed to 197Pt formed from 196Pt by neutron absorption.[64] Direct detection of neutron emission has not been consistent.[65] Production of neutrons and subsequent activation of Pt might be caused by occasional cracking with the resulting fractofusion. Consequently, the emitted neutrons may have nothing to do with an anomalous nuclear reaction. With Mizuno's help, Oriani[66] was able to duplicate production of AE. A similar complex oxide was studied by Samgin et al.[67] and it also was found to produce AE.
Biberian et al.[68] applied the same method to LaAlO3 and reported detecting AE. Single crystals were not active.
QUESTION #5. WHY IS THE EFFECT SO DIFFICULT TO REPRODUCE?
Failure to replicate these claims is frequently attributed to errors in the successful work that are not present during the unsuccessful studies. Thus, the failed studies are considered to be well done and correct, while successful studies are thought to be deficient in some way. Ironically, while some famous failed studies were considered well done at the time, later analysis revealed actual production of anomalous energy.[69-73]
A better explanation attributes failure to various properties of the materials being used, which have nothing to do with error although some error is certainly present. This explanation shifts attention away from possible sources of error and instead emphasizes how the cathode material was treated. As will be shown below, this approach has considerable experimental support, especially for the P-F method.
|
|
|
|
|
Boron Containing Samples made at NRL |
|
|
|
J-M Pd |
|
|
|
NRL Pd |
|
|
|
WESGO Pd |
|
|
|
NRL Pd-Ag |
|
|
|
IMRA Pd-Ag |
|
|
|
Pd-Cu |
|
|
|
Pd-Ce |
|
|
|
Co-deposition |
|
|
(J-M: Johnson and Matthey Company
NRL: Naval Research Laboratory
MRA: IMRA Japan
Co-deposition: Pd plated from solution during heat measurement
WESCO: A secondary supplier used early in the work)
Some of the methods listed in Table 1 are easily to reproduce and some are not. In most cases, the reasons are not yet known. Unfortunately, the method chosen by P-F has been especially difficult to reproduce because certain properties of palladium can not be duplicated from sample to sample. For example, most of the time, this metal forms cracks when it reacts with hydrogen or deuterium[44, 74, 75], a process that opens paths through which dissolved deuterium can escape as D2 gas. As a result, the cathode can not achieve the required high deuterium content. Everyone who has studied the effect using bulk palladium has discovered certain batches to be more likely to work. Successful material has been shown not to form such cracks. One example of this experience is shown in Table 3.[76] Another example is provided by the experience of Storms[44], Kobayashi et al.[77], and Miyamaru and Takahashi [78]. Each of these workers used palladium from the same batch prepared by Tanaka metals (Japan) and each found AE. This palladium was especially free of cracks. A subsequent batch containing many cracks was found to be dead by the same people. Thus, when the same batches were studied using different equipment by three independent laboratories, the same behavior was observed.
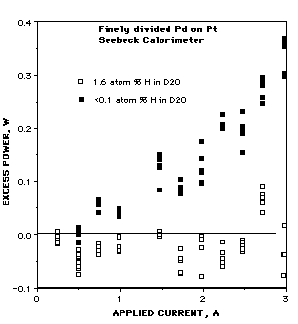
FIGURE 5. Effect of placing an active cathode in an electrolyte containing H2O. The same calorimeter was used for both measurements. This work was done at the author's laboratory in Santa Fe, NM.
Minor impurities such as light water [79, 80], as shown in Fig. 5, kill the effect. This observation is important for three reasons. First, it is hard to imagine how so little H2O in the D2O can remove those errors thought by critics to have produced an apparent excess energy before H2O was added. Second, this effect means that p-d fusion does not contribute to energy production. Third, this is one more reason why the effect has not been easy to duplicate. Heavy water quickly absorbs light water from the atmosphere unless the cell is sealed, a rare precaution during early studies. Dissolved impurity metals in the electrolyte can also quickly kill the effect. In fact, commercial D2O has been found to contain up to 9 ppm of impurity, material that must be removed by distillation or by pre-electrolyzing with a dummy cathode [44].
These observations suggest that the factors preventing duplication of the P-F effect are associated with the properties of bulk palladium and impurities within the electrolyte. On the other hand, once a piece of palladium has made AE, most people find this particular piece of metal to be active every time it is studied, unless the surface layer is altered [81] or unless cracks begin to form, something repeated reloading will encourage[82]. For example, Miles [83] took a piece of palladium found to be active in his laboratory at China Lake in the US to the NHE laboratory in Japan, where it again was found to be active. Other people frequently find this kind of reproducibility within their own laboratories.
Thin layers of palladium deposited on an inert substrate make AE more easily than does bulk palladium.[61, 84, 85]. Therefore, a person wishing to replicate the claims of P-F should use this approach. Other electrode materials are less susceptible to their bulk properties, although surface conditions are still important.[51, 61, 86-90].
If anomalous energy is accepted as being real, the next question is:
QUESTION #6: DOES AE HAVE A NUCLEAR SOURCE?
Three types of answers are offered. First, the amount of energy is too great to be explained by any other process. This argument is weak because the amount of energy depends on how long the sample appears to make AP. If an offset error is present, a large amount of apparent AE can be created simply by being patient. Second, no chemical products have been found to accumulate in cells making AE. This argument is stronger than the first one, but still relies on not finding something that available techniques might miss. Third, nuclear products are seen to accumulate within the cell in amounts consistent with the amount of AE being measured. This is the strongest argument, but the most difficult to demonstrate because very small quantities of material are involved, some of which might well come from conventional sources.
Possible products of a fusion reaction are neutrons, tritium, charged particles, gamma emission and 4He. All of these nuclear products have been looked for and found. Because the first two products result from "normal" fusion, they were frequently sought in early studies. Recent studies have looked for elements resulting from transmutation reactions, a process that has the potential to produce a wide spectrum of nuclei.
Neutrons: Because neutron emission is easy to detect, at least 500 attempts were made to find this product. All of this effort has revealed that neutrons are not emitted from CANR cells in amounts consistent with the AE. When neutrons are found, they are emitted when AE is produced and when it is not. In either case, the measured energy is 2.54 MeV and up. The behavior would suggest that fractofusion [91-94] is occurring within the deuterium containing metal. This process occurs when a crack forms and generates a large voltage gradient. This voltage can accelerate D+ ions and produce normal, high-energy fusion. Because cracks form easily in palladium [75, 95], it is impossible to know whether the few detected neutrons are emitted only from the anomalous cold fusion reaction or result from localized "normal" fusion. In addition, other reactions having nothing to do with fusion might also be the source under special conditions. In any case, neutron emission, when it occurs at all, is from a minor reaction path.
Mizuno et al. [96] have proposed that a mixture of light and heavy hydrogen enhances neutron emission during the electrolytic process. They observe most neutron bursts to occur after considerable electrolysis has taken place, when H2O has had a chance to enter the D2O electrolyte. To test this idea, they loaded a Pd electrode in D2O and then transferred the cathode to a cell containing H2O. Neutron bursts frequently occurred shortly after electrolysis was started and when the cell voltage was increased, thereby providing more H2 to the cathode. The improved reproducibility and magnitude of neutron emission might explain why neutron emission is seen so infrequently when efforts are made to keep the electrolyte free of H2O. However, this behavior is not inconsistent with fractofusion because loading with hydrogen, especially at high-applied voltage, will stress the material more than when deuterium alone is used, hence will produce more cracking. However, if neutrons at 2.54 MeV are found to be absent, another process would be indicated.
Tritium: Over 200 attempts have been made to detect tritium with about 24 reporting this product, sometimes at significant levels, but not enough to account for the AE. Apparently, the tritium-producing reaction is rare and difficult to initiate. Three particularly compelling results have been published using the P-F method, a low-energy gas discharge, and gas loading of palladium-black.
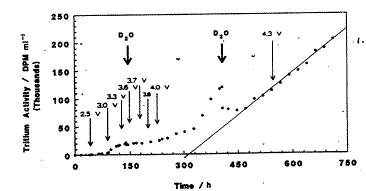
FIGURE 6. Tritium concentration as a function of time found in a Pons-Fleischmann-type cell being electrolyzed at the indicated voltage. Heavy-water was added to the electrolyte at the indicated times. This work was done at Texas A & M University.
Dr. Chien et al. (Texas A & M) [97] measured the tritium content of an open electrolytic cell containing a Pd cathode and LiOD in heavy water. They found the tritium content of the electrolyte was influenced by changes in the cell current (overvoltage) and was a linear function of time when conditions were constant, as shown in Fig. 6. However, shaking the cell caused tritium production to stop. It is hard to imagine how this simple act could influence tritium pickup from a prosaic source. On the other hand, it suggests that tritium production is associated with surface features that are easy to shake off. Taubes [98] claimed that tritium was being added by a student. This accusation was not supported by an investigation at the university. In addition, thirty-five tritium additions would be needed, each at precisely the correct time and in precisely the correct amount, a feat very hard to keep undetected at a university where people come and go at will. Other people have suggested tritium was present in the palladium cathode and was slowly released by electrolytic action. To counter this argument, Storms[82] used the same technique to study a sample of Pd known to contain tritium. He found that tritium, which was dissolved in the metal, appeared in the evolving gas rather than in the electrolyte where it was detected by Chien et al. This work demonstrated that the tritium claimed by Chien et al. could not result from tritium contamination. The study by Storms also demonstrates that anomalous tritium originates at the surface where it can exchange with the surrounding electrolyte rather than being lost as DT gas, as would be the case if it formed within the bulk material. In addition, a very complete analysis for tritium in palladium metal, obtained from many suppliers, shows that palladium from commercial sources simply does not contain tritium.[99, 100] Tritium enrichment is also a possible source of increased tritium concentration because deuterium is lost faster from such cells than is tritium. The enrichment factor is known [101-103] and can not account for all of the tritium found in this or other studies.
Dr. Claytor [104, 105] and co-workers have studied tritium production at the Los Alamos National Laboratory for many years using a pulsed discharge method in low-pressure D2 gas. The voltage is too low to produce tritium by conventional processes. Tritium is measured in real time using an ionization detector within the gas line and later as total tritium content by converting the gas to D2O. Tritium contained in the resulting D2O is measured using scintillation counting. Both techniques are very sensitive and show good agreement. A variety of alloys have been studied which show that tritium production is very sensitive to the material used for the cathode. Although the amounts produced are small, no prosaic explanation has stood the test of detailed examination by many reviewers, both inside and outside the laboratory.
Arata and Zhang [106] as well as McKubre et al. detected He-3 in the D2 gas after excess energy was made. This He-3 was found to result from decay of tritium. The amount of He-3 combined with the decay rate showed that the tritium was created at the same time EP was being produced. However, the amount of tritium was much too small to contribute significant energy to the process.
Each of these studies suggests tritium forms on nanosized particles. Such locations being rare and difficult to form, tritium production is seldom detected. Why an isotope like tritium, i.e. one containing excess energy, should form in this environment instead of the ground-state (3He) is a major challenge for any theory.
Helium: If these products are not the source of AE, what is? Helium-4 is claimed to result when AE is produced. However, formation of 4He is expected to generate gamma emission. Gamma emission from a CANR cell, although detected sometimes at low levels, is not consistent with the amount of 4He detected. For this inconsistency to be resolved, the presence of 4He needs to be proven. Several problems make this difficult. Air contains about 6 ppm of helium, making an air leak a possible source of anomalous helium. Of course, a leak will not add this much helium to the contained gas unless all of the gas in the apparatus is air. Clearly all of the deuterium could not be replaced by air without this fact being recognized. Absence of argon in the gas eliminates an air leak as an explanation for detected helium. Most studies have failed to detect argon in the D2 gas. Even preferential diffusion of He through the walls would not result in the air concentration being achieved within the apparatus in any reasonable time. Therefore, detecting He-4 even close to the air concentration would be proof that the He resulted from some process within the apparatus.
A possible source of helium within the apparatus is that which is dissolved in or absorbed on the cell components. Studies have shown that helium dissolved in palladium deeper than a few microns can not be removed unless the material is almost melted.[107] Therefore, this source is precluded when helium is found in a P-F cell. Charcoal, on the other hand, can absorb helium at room temperature and above, and this can be removed by flushing with hydrogen. Consequently, care must be used when examining a charcoal containing material for helium production.
Numerous samples of palladium have been examined for their helium content after having produced AE, with mixed success . On the other hand, four studies have shown a quantitative relationship between helium found in the surrounding gas and AE production.
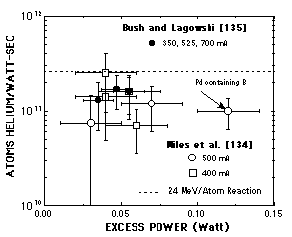
FIGURE 7. Relationship between atoms of helium per watt-sec of energy and anomalous power generated in a Pons-Fleischmann cell. Helium was measured in the generated gas and does not include helium retained by the palladium cathode. This work was done at the Naval Air Warfare Center at China Lake and at SRI.
Miles et al.[108-110], working at the Naval Air Warfare Center (China Lake), recovered gases being generated in a P-F type cell while making AE and measured the amount of contained 4He. A double-wall isoperibolic calorimeter was used and gas was collected in metal flasks. An earlier study [111] using glass flasks is ignored here even though the result is consistent with their latter work. Six samples are reported to make AE along with helium at levels above background. Five samples showed no AE and the helium concentration was at background. Bush and Lagowski[112] repeated this study using a Seebeck calorimeter, a different stainless steel apparatus, and at a different laboratory (SRI). These results are compared in Fig. 7. Jones and Hansen [113] criticized the Miles work for confusion in reporting some values, for not running proper controls, for poor calorimetry, and for not measuring possible recombination properly. Their latter criticism is shown not to be valid in a previous section. Miles responded first in a question and answer secession [114] and then in a formal publication.[115] Jones et al. [116] had the last word after much of Miles' response was omitted by the journal. While the issues were not resolved and remain clouded in misunderstanding, a relationship between heat production and helium production has a high probability of being real. Gozzi et al. [117, 118] monitored the real-time production of 4He from an active cell using an inline mass spectrometer. Although some problems with air leaks were acknowledged, they demonstrated a clear relationship between energy and helium production. When these three studies are added to the helium-energy values reported by McKubre et al., as shown in Fig. 4, the reality becomes even stronger. Apparently the nuclear reaction producing helium has an energy close to that expected from d-d fusion. The insistence that gamma emission must accompany helium production is based on how this fusion branch behaves in a plasma. Because the reaction d + d = 4He has two nuclei producing one nucleus, gamma radiation must occur to conserve momentum. On the other hand, suppose the following reaction occurs in a lattice where the d concentration is very high[119] [120]:
d+d+d+d = {8Be} = 2 4He.
Such a reaction would not require gamma emission because 8Be would promptly decompose into two particles, each having 23.8 MeV. Other, similar reactions can be proposed to avoid the need to emit gamma radiation. This suggestion shifts the problem from requiring gamma emission, to accepting that such reactions can actually occur. Evidence for such multibody interaction has been reported by Takahashi et al.[121] based on the energy of tritons emitted when titanium is bombarded by D+.
Transmutation: Transmutation describes a nuclear reaction involving nuclei heavier than hydrogen. Such reactions occur when hydrogen or a heavier nucleus enters another heavy nucleus causing it to transmute (change) to another element. Because the Coulomb barrier is very high for such reactions, significant energy is normally required. For this reason, claims for such reactions in the CANR environment are surprising and difficult to explain without invoking prosaic processes or error. If true, these observations imply that the Coulomb barrier, no matter how large, can be reduced to insignificant values by a process available within a solid.
Transmutation reactions have been reported to occur in all environments to which the CANR process has been applied. The easiest method involves creating a plasma under water. This can be done by applying sufficient voltage (up to 150 V) to form an arc between two carbon rods immersed in an electrolyte containing various salts dissolved in water [122-124]. The method is reported to generate a magnetic precipitate in addition to various elements and is easy to duplicate. A tungsten electrode can also be used[125-127] or the discharge can be made to occur in a cell made of zirconium[128, 129]. Each has been reported to generate elements not previously detected in the materials, sometimes with abnormal isotopic abundance. The most complete study was undertaken by Prof. Miley [130-132] using electrolytic current applied to a nickel cathode in H2O-based electrolyte. Figure 8 shows the rate of element formation as a function of atomic number. Many of the observed elements also had an abnormal isotopic ratio. Of course, some of these elements might have resulted from contamination or from errors in chemical analysis. On the other hand, Ag and Cu exceed potential contamination by several orders of magnitude. The number of elements found and the pattern shown by the data are hard to explain based on prosaic sources.
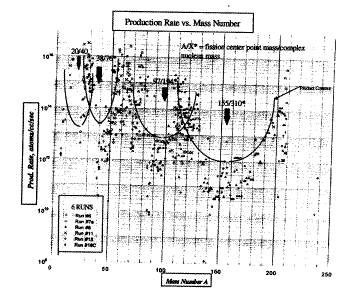
FIGURE 8. Rate of element production in an electrolytic cell using a Ni cathode and a H2O-based electrolyte as a function of atomic number of the element produced. This study was done at the University of Illinois.
Transmutation is claimed to occur in living systems where the process becomes especially difficult to believe or understand. Indeed, people have advised me not to discuss this subject, as if a potential reader would be too immature to handle the intellectual conflict this discussion might cause. Readers are warned to skip this section if such information would damage their open mind.
A scientist in Japan[133-137] started the process to duplicate claims made popular [138] by Kervran [139] and published the results first in France. He used modern analytical methods to study eight different biological cultures of bacteria and yeast, each made deficient in K, Mg, Ca, or Fe. The growing cells are found to make the deficient element from the other elements present. The following reactions are proposed:
23Na +16O = 39K
23Na + 1H = 24Mg
39K + 1H = 40Ca
24Mg + 16O = 40Ca
28Si + 12C = 40Ca
Later, Russian workers started a similar study[140, 141]. This team first made cultures of three different bacilli and one yeast, all of which could live in either D2O or H2O. The cultures were made iron-free, but contained a small amount of MnSO4. After a suitable time, the presence of 57Fe was measured using the Mössbauer effect. Iron 57 was only detected when both MnSO4 and D2O were present in the cultures. The proposed reaction is 55Mn +2D = 57Fe. The rate of growth was measured and was calculated to give no more than 50 mW to the growing media. Of course, the culture containing H2O would be expected to make 56Fe, which would be invisible to the Mössbauer method. It is interesting that Kervran [139] published a study over forty years ago in which MnSO4 placed in a culture growing in H2O made iron that was easily observed. Naturally, this work was ignored. The Russian team has filed a patent for the process. The team has now explored the reaction 23Na + 31P = 54Fe. A time of flight mass spectrometer was used to determine the concentrations of reactants and product. They report that a culture made deficient in iron would generate iron only when both Na and P are present.
QUESTION #7: HOW CAN THE CLAIMS BE EXPLAINED?
Objections to cold fusion being real rely on the following arguments. First, a large application of energy is required to overcome the Coulomb barrier. If a nuclear reaction should occur, the immediate release of energy can not be communicated to the lattice in the time available. When such energy is released under "normal" conditions, energetic particles are emitted along with various kinds of radiation, only a few of which are seen by CANR studies. In addition, gamma emission must accompany helium, and production of neutrons and tritium, in equal amounts, must result from any fusion reaction. None of these conditions is observed during the claimed CANR effect, no matter how carefully or how often they have been sought. Furthermore, many attempts to calculate fusion rates based on conventional models fail to support the claimed rates within PdD. The atoms are simply too far apart.
Failure to discover just what the nuclear-active-environment really looks like is a major problem in answering some of these challenges. A few observations suggest where to look. When the electrolytic method is used, AE is produced on the surface within small, isolated regions.[84, 142] These regions heat up in a random fashion, thereby losing the required deuterium content, which stops further reaction. Thousands of such local regions flash on and off, adding their resulting energy to the total. This process is self-regulating and usually produces a smooth generation of heat energy. Occasionally, a region will generate enough energy to cause local melting[143, 144]. Once in awhile, large bursts of total energy are observed. The number of such regions on the surface determines just how much total power will be produced. This description implies that power density in the active regions is extremely high and has no relationship at all to the often quoted power density based on the physical volume of the sample.
(Note: some browsers cannot display characters in the symbol font. If betas do not appear in the following, read beta-PdD for b-PdD wherever it occurs in the following).
While the nature of these regions is still unknown, they are clearly not b-PdD [145], as most people have assumed when the P-F method is used. Not only is the active composition in excess of PdD1.0 [146-148], the upper composition limit for this phase, but deposition of various impurities makes the surface a complex alloy [149-155]. Neither the structure nor the composition of this alloy is known. In fact, some nuclear-active materials may not contain palladium at all.
A growing number of materials, besides palladium, are found to be useful for the P-F method, with bulk palladium being the most difficult to make active. Two conditions appear to be required: the ability of the material to acquire a high deuterium content and the size of the active regions. The required size appears to be less than 1 µm. A high flux of deuterium through the material also seems to be beneficial [156-158], although not necessary. The active material will be equally difficult to locate and identify when other methods, besides electrolysis, are examined.
In summary, the active material within a P-F cell is on the surface and located in very small, isolated regions. Bulk properties have little relationship to the properties of these regions, which consist of a complex alloy having very little in common with b-PdD. Very little progress will be made until the proposed models are applied to the real world rather than using the ideal environment within b-PdD. In addition, bulk properties can not be used to support a model or to reject the claims.
Ordinary hydrogen produces both AE and NP using the same methods applied to deuterium. Obviously, fusion can not be a source of either product. In addition, the materials required to initiate anomalous effects are different between the two isotopes. For example, palladium is found to be nuclear-active with deuterium while only nickel is found to be active with hydrogen. As expected, the types of nuclear reactions are different, as well as the environments in which they occur. This difference suggests several different mechanisms may be operating. Such a possibility needs to be considered when evaluating the proposed models.
Once the environment has been created, what process might allow a nuclear reaction to take place? Only a few proposed mechanisms have been developed in sufficient detail to be useful or to allow an evaluation. A useful model must address more than just the conditions thought to exist when the P-F method is used and must consider the following:
#1. describe how the Coulomb barrier is overcome,
#2. show how the released nuclear energy is distributed throughout the atomic lattice,
#3. show why and how different environments produce different nuclear reactions, and
#4. recognize the unique characteristics required of a nuclear-active environment.
Proposed theories can be evaluated with respect to the initial assumptions, to the accuracy of the applied mathematics, and to how well the theory treats the above conditions. Only the latter evaluation will be applied here. Of course, many assumptions used to meet these conditions may not be considered reasonable. This part of the evaluation is left for the reader, although a few evaluations at this level have been published.[159-161]. The following discussion must, for space considerations, be very simple and omit many efforts to explain the effect. The author apologizes in advance for giving so much hard work so little attention.
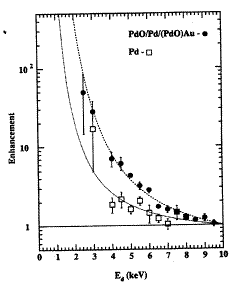
FIGURE 9. Enhancement over the effect found at high energy for the reaction D(d,p)T as a function of energy applied to D+ used to bombard the indicated targets. This work was done at Tohoku University, Japan.
Enhanced Cross Section: The observations imply a higher than expected cross-section for fusion at very low energy. To explore this possibility, Kasagi et al.[162] bombarded various metals and compounds with deuterons of various energies down to 2.5 keV and measured how much enhancement was produced in the reaction D(d,p)T, as shown in Figure 9. Apparently, an increase in cross-section does occur at low energy when the reaction occurs in a solid, in contrast to when the reaction is initiated in a plasma. In addition, enhancement is greater in PdO than in a number of pure elements[163], thereby showing that the nature of the chemical environment is important. Because the solids were not expected to be nuclear-active under the conditions of the study, the measured cross-sections represent only a lower limit for what might be possible when the "correct" solid is used. Other studies indicate that the branching ratio between tritium and neutron production might also change at low energy.[164-166] Once these possibilities are acknowledged, the next problem is to propose how the cross-sections might be increased at low energy by the surrounding atoms.
Neutron Formation: Neutrons are assumed either to be present in the lattice within a stabilizing structure [167] or are created by collapse of an electron [168-177] into the nucleus of a hydrogen or deuterium. The latter collapse makes a dineutron.[178, 179] The importance of a neutron presence is suggested by the unusual effects observed when an external neutron flux is applied to a CANR cell. [180-184] Apparently, the environment acts like a neutron amplifier. This model answers only Requirement #1. In addition, one might ask why more neutrons are not detected as they are being released or created within the cell, especially when thin cathodes are used?
Fisher [185] [186] has proposed that large, stable neutron clusters can form and that these can attach themselves to normal nuclei to produce super-heavy atoms. A small concentration of such atoms is proposed to be present in all matter. Under the right conditions, these neutron clusters are released, thereby causing novel nuclear reactions. The work of Oriani [187] supports the existence of super-heavy carbon in electrodes subjected to CANR processes.
Particle-wave Transformation: Talbot and Scott Chubb (uncle and nephew) [188-190] have explored the possibility of particle-wave conversion, a process known to occur at very low temperatures. In this model, deuterons convert to waves in the correct environment and these waves interact to form a helium wave within the surrounding atomic lattice. Once sufficient energy has been lost by the d-d-He wave packet, it becomes a 4He particle. In their model, a periodic lattice of suitable size containing a very high concentration of deuterium nuclei is required. Helium already present within the structure and a deuteron flux are proposed to be beneficial. Liboff [191, 192] has also explored this theme in a different way.
This model answers Requirements #1, #2 and #4 very well, but answering #3 requires some less attractive assumptions. Also, one might wonder why the proton is not also converted to a wave and, as such, combine with a deuteron wave to make 3He, an isotope not detected except as the decay product of tritium.
While not using the vocabulary of particle-wave conversion, Hagelstein[193] has arrived, after several detours, at a model he proposes answers all of the required conditions. He assumes phonons can interact with the nucleus and these interactions can be at different sites in a lattice, yet coupled together to produce the observed result. In this model, phonons provide the mechanism for coupling rather than wave formation of atoms. Of course, a phonon, which is a mathematical construct used to describe thermal vibrations, must accumulate considerable energy either before reacting or within the nucleus[194] after reacting, two precesses that are hard to justify.
Resonance, Tunneling and Screening: Deuterons in a lattice experience thermal vibration and, by this process, might get close enough to interact with each other or with the palladium nuclei. However, many calculations show that the atoms are too far apart in b-PdD to interact. If this type of interaction is to occur, neutralization of the Coulomb barrier by surrounding electrons (screening) and/or enhanced barrier penetration using a process called resonance tunneling, as first suggested by Turner[195], must be applied to the process. Each aspect of this process has been addressed in different ways. Agreement is far from universal that any variation on this theme, of which there are many, can explain the amount of AE observed. Only a few will be described here to give a general understanding of the methods being applied.
Several people [196, 197] [198] have reformulated a calculation of the Gamow factor which defines how repulsive forces change with distance between nuclei and determines how much "normal" tunneling might take place. By applying a resonance process, a higher than expected fusion rate was calculated, consistent with the Jones [199] level of neutron emission, but not high enough to explain the P-F heat. As noted above, the neutron flux measured by Jones may not have originated from a CANR process at all, but be caused by fractofusion. Clearly, for such a theory to be useful, additional processes must operate. Parmenter[200] comes to the conclusion that "tunneling is most likely when the energy of the deuteron pair most closely matches that of the 4He isomer. Pulsed loading is suggested as a way to achieve this condition. This process may operate unintentionally as the active regions experience changes in loading produced by bubble action. However, this mechanism would not apply to the other methods.
Preparata[201] imagines a coherent plasma of electrons within the PdD structure that are able to off-set the charge at the nucleus. He assumes b-PdD exists only in the range PdD0.6-0.7 while another phase (gamma), based on tetrahedral occupancy, forms at higher compositions. Unfortunately, the model involves a faulty understanding of the Pd-D system [202] because tetrahedral occupancy has never been observed in this system [203-205] even when its presence was sought. To add to the confusion, a previously designated gamma phase is seen only below 50 K at PdH0.67 as an ordering of deuterium vacancies [206]. The very large compositions reported for the surface of PdD, as noted above, suggests a new phase (PdD2)[145] to which the model might be applied. Lo [207] has also explored this theme.
Exotic Particles: Several unusual particles are proposed to catalyze nuclear reactions. Such an explanation may answer Requirement #1 in an incomplete way but, in most cases, fails to address the other requirements. However, the implications go well beyond the CANR effect if the claims are true.
Bazhutov et al. [208-210] have proposed the existence of a heavy hadron (175 GeV/c2) called Erzion, which they have identified in cosmic rays. In a similar manner, but independently, Rafelski et al.[211] visualize a stable, ultra heavy, negatively charged particle called X- to rain down from outer space. These models do not address Requirements #2, #3 or #4.
McKibben [212, 213] proposes the existence of three sets of fractionally charged particles called subquarks (1.15 amu, charge = +e/6), hemitrons (0.13 amu, charge =+e/2, and negative hemitrons (>0.13 amu, charge = -e/2). These are proposed to form into nuclei and mimic the properties of normal isotopes. However, because these pseudo-nuclei are more unstable than their normal counterpart, they can enter into and catalyze nuclear reactions more easily. This model concludes that nuclear-active material contains more of these pseudo-elements than does inactive material. Why the pseudo-elements should be so highly localized and why such a long delay exists for the production of AE are not addressed.
Formation of proton or deuteron clusters: Dufour et al.[214, 215] have observed unusual behavior when a low current-high voltage discharge occurs in H2 or D2. These authors have now developed a model based on clusters involving a large number of protons or deuterons combined with the required number of electrons. These clusters, called hydrex and deutex, can bind to a nucleus, thereby lowering the Coulomb barrier for various nuclear reactions.[216, 217] A variety of nuclear products have been found that depend on the materials placed in the discharge, as well as production of anomalous energy. Matsumoto[218] also proposes a deuteron cluster (Nattoh model) in which the fusion reaction is thought to occur. A new particle called Iton is proposed to be emitted from this reaction. Very strange tracks are found in photographic film placed near an active cell, which support this and other interpretations. [219, 220] These models answer only Requirement #1
Formation of electron clusters: Millions of electrons are proposed by Shoulders [221] to form into clusters during any spark discharge. These clusters interact with solids to produce local regions having very unusual properties. This proposal is based on years of study, the details of which have yet to be published, although patents have been issued. [222] When these electron clusters encounter a dielectric, they strongly interact to produce many effects including nuclear reactions. This concept has been applied by Fox et al.[223-225] to a number of conditions used to initiate the LENR effect. Lewis [226] has also proposed similar ideas. Some of the strange tracks found in film placed near a CANR cell [220] may be produced by such charge clusters. [224]
3. CONCLUSION
A growing number of successful replications of anomalous energy using different methods are being reported. This energy is clearly related to the nature of the environment and appears to be generated by various nuclear reactions. Two questions need answers. Do these observations show a real and novel feature of nature? If the observations are truly novel, then how does the nuclear active environment differ from ordinary matter, which is clearly inert? Which of the various observed nuclear reactions is favored also depends on the environment. This being the case, materials science plays a role as important as does nuclear physics.
Evidence for the reality of the claims comes from three sources. First, most of the methods that claim to produce anomalous effects have been duplicated, as noted in Table 1 and some have been replicated many times, especially the PF effect as listed in Table 2. As the reader can plainly see, this fact is in sharp contrast to popular perception [227]. Nevertheless, replication is not always successful, requiring knowledge of the important variables and some luck in acquiring special materials. Thanks to the persistence of a few workers, replication is now much easier than was initially the case. Second, similar patterns of behavior have been observed by every investigator who bothered to make the necessary measurement. As an example, a universal relationship exists between the average deuterium content of the cathode and the amount of AP observed using the P-F effect. Third, a study of the D(d,p)T reaction using conventional ion implantation techniques shows that the cross-section for the reaction becomes greater the lower the bombarding energy below 2.5 keV and it is sensitive to the chemical environment. While the measured cross-section is too low to explain the observed EP, one might wonder what would be observed at energies near room temperature when a nuclear-active environment is used.
While many environments, including both metals and compounds, have been successful in hosting AP and NP production, the most successful common feature is the size of the active domain. Crystals having dimensions in the region between micro- and nanometers appear to be the most active. Unfortunately, these domains are frequently not part of the characterized bulk material, but are deposited by various processes on the surface. For example, they can be deposited by electrolysis when the P-F method is used or by ion bombardment during gas discharge. Once formed, these microregions need only to acquire deuterium or, in some cases, hydrogen to initiate a nuclear reaction. In addition to size, a flux of hydrogen or deuterium appears to be necessary. This flux can be generated using either a temperature or concentration gradient. The presence of other elements in the active environment add complexity to the resulting nuclear products.
Apparently, even large Coulomb barriers appear to be overcome by processes currently being explored. Although the mechanism is still not completely understood, no new physics appears to be necessary. Nevertheless, nuclear behavior is quite different from that experienced when high energy is used to initiate such reactions. Especially novel is the absence of radioactivity and energetic radiation, including neutron and gamma emissions. The challenge is to find a rational explanation. This explanation can not be found unless the observations are made known to the general scientific profession, thereby allowing a broad range of knowledge to be applied.
The large number of nuclear reactions being reported and the types of required environments give a particular challenge to theoreticians. In contrast to conventional experience based on using high energy to overcome the Coulomb barrier by brute force, the CANR environment apparently uses a mechanism that can neutralize the barrier. This more subtle method apparently is obscured when high energy is applied, this situation being like the difference between a rape and a seduction. The problem is to identify the nature of these environments. Up to now, almost all effort has been focused on explaining how the nuclear reactions can take place once the environment is created. While this insight is important, it has not been much help in finding the best environments. This approach needs to change if commercial applications are to be achieved and if the skeptical attitude is to change.
Everyone who has worked in this field has suffered an amount of professional rejection and personal attack well out of proportion to the scientific issues.[228-231] Professors Stanley Pons and Martin Fleischmann paid an especially high price at the hands of their fellow scientists. This attitude has even extended to the US Patent Office. With only a few exceptions, patents cannot be obtained in the US on this subject, although other countries issue patent protection on a routine basis. This rejecting attitude has also been adopted by industry and by most levels of government, especially the U.S. Department of Energy. In addition, a few individuals have repeatedly intervened to prevent an open and honest discussion of the subject, as described by Mallove and Rothwell [232]. In view of the expected energy shortage, the threat of global warming, and the growing accumulation of nuclear waste, the present attitude needs to change [233]. This phenomenon has the potential to solve several major problems if normal procedures for scientific discussion and study are allowed. In spite of this general rejection, the American Physical Society, the American Chemical Society, and the American Nuclear Society are to be complimented for their willingness to permit discussion of this subject at their recent conferences. Fusion Technology, a publication of the American Nuclear Society, edited until recently by George Miley, and the Journal of Electroanalytical Chemistry have been especially helpful in the past. However, Physical Review B, Review of Modern Physics, Chemical Reviews, and J. Electroanalytical Chemistry turned down a request to publish this review. Fusion Science and Technology (formerly Fusion Technology), is also unwilling to publish papers on the subject.
References (those for table 2 are listed separately)
1. Fleischmann, M., S. Pons, and M. Hawkins, Electrochemically induced nuclear fusion of deuterium. J. Electroanal. Chem., 1989. 261: p. 301 and errata in Vol. 263.
2. Pons, S. and M. Fleischmann, Calorimetric measurements of the palladium/deuterium system: fact and fiction. Fusion Technol., 1990. 17: p. 669.
3. Fleischmann, M. and S. Pons, Some comments on the paper Analysis of experiments on the calorimetry of LiOD-D2O electrochemical cells, R.H. Wilson et al., J. Electroanal. Chem. 332 [1992] 1. J. Electroanal. Chem., 1992. 332: p. 33.
4. Fleischmann, M. and S. Pons, Calorimetry of the Pd-D2O system: from simplicity via complications to simplicity. Phys. Lett. A, 1993. 176: p. 118.
5. Fleischmann, M. and S. Pons, Reply to the critique by Morrison entitled 'Comments on claims of excess enthalpy by FLeischmann and Pons using simple cells made to boil. Phys. Lett. A, 1994. 187: p. 276.
6. Britz, D., Bibliography of the field, http://www.chem.au.dk/~db/fusion
7. Fox, H., Bibliography of the field, Fusion Information Center, (801) 466-8680.
8. Mallove, E., bibliography of the field, Cold Fusion Technology, (603) 228-4516.
9. Storms, E., Reviews,, http://home.netcom.com/~storms2/index.html.
10. Miles, M.H., B.F. Bush, and D.E. Stilwell, Calorimetric principles and problems in measurements of excess power during Pd-D2O electrolysis. J. Phys. Chem., 1994. 98: p. 1948.
11. Wagner, F.T., et al., A comparison of calorimetric methods applied to the electrolysis of heavy water on palladium cathodes. J. Electroanal. Chem., 1990. 295: p. 393.
12. Hansen, W.N. Report to the Utah State Fusion/Energy Council on the Analysis of Selected Pons Fleischmann Calorimetric Data. in Second Annual Conference on Cold Fusion, "The Science of Cold Fusion". 1991. Como, Italy: Societa Italiana di Fisica, Bologna, Italy.
13. Shelton, D.S., et al., An assessment of claims of 'excess heat' in 'cold fusion' calorimetry. Thermochim. Acta, 1997. 297: p. 7.
14. Miskelly, G.M., et al., Analysis of the published calorimetric evidence for electrochemical fusion of deuterium in palladium. Science, 1989. 246: p. 793.
15. Case, M. and R. Boehm. Assessment of thermal energy output from electrochemical cells - a critical review. in HDT (Am. Soc. Mech. Eng.) 151 (Heat Transfer Adv. Energy Syst.). 1990.
16. Sioda, R.E. and T.Z. Fahidy, A simplified approach to the thermal behaviour of electrolytic Dewar cell calorimeters. J. Appl. Electrochem., 1992. 22: p. 347.
17. Jones, J.E., et al., Faradaic efficiencies less than 100% during electrolysis of water can account for reports of excess heat in 'cold fusion' cells. J. Phys. Chem., 1995. 99: p. 6973.
18. An, X.-W., et al., Calorimetric investigation of electrochemically induced nuclear fusion of deuterium. Thermochim. Acta, 1991. 183: p. 107.
19. Lewis, N.S., et al., Searches for low-temperature nuclear fusion of deuterium in palladium. Nature, 1989. 340(6234): p. 525.
20. Storms, E., Description of a dual calorimeter. Infinite Energy, 2000. 6(34): p. 22.
21. Langmuir, I., Pathological Science. Physics Today, 1989. October: p. 36.
22. Groenlund, F., Electrolysis in calorimetry. J. Thermal Anal., 1992. 38: p. 229.
23. Keddam, M., Some comments on the calorimetric aspects of the electrochemical 'cold fusion' by M. Fleischmann and S. Pons. Electrochim. Acta, 1989. 34(7): p. 995.
24. Morrison, D.R.O., Comments on claims of excess enthalpy by Fleischmann and Pons using simple cells made to boil. Phys. Lett. A, 1994. 185: p. 498.
25. Ohashi, H. and T. Morozumi, Decoding of thermal data in Fleischmann & Pons paper. J. Nucl. Sci. Technol., 1989. 26(7): p. 729.
26. Speiser, B. and A. Rieker, Energy from electrochemically induced nuclear fusion? Nachr. Chem. Tech. Lab., 1989. 37: p. 616 (in German).
27. Wilson, R.H., et al., Analysis of experiments on the calorimetry of LiOD-D2O electrochemical cells. J. Electroanal. Chem., 1992. 332: p. 1.
28. Hansen, W.N. and M.E. Melich, Pd/D Calorimetry- The Key to the F/P Effect and a Challenge to Science. Trans. Fusion Technol., 1994. 26(4T): p. 355.
29. Saito, T., et al. Studies on Fleishmann-Pons Calorimetry with ICARUS 1. in 5th International Conference on Cold Fusion. 1995. Monte-Carlo, Monaco: IMRA Europe, Sophia Antipolis Cedex, France.
30. Ohms, D., D. Rahner, and K. Wiesener, Kernfusion in einer Elektrolysezelle?" ("Nuclear fusion in an electrolysis cell?"). Mitteilungsblatt - Chem. Ges. DDR, 1989. 36: p. 151 (in German).
31. Swartz, M.R. Some Lessons From Optical Examination of the PFC Phase-II Calorimetric Curves. in Fourth International Conference on Cold Fusion. 1993. Lahaina, Maui: Electric Power Research Institute 3412 Hillview Ave., Palo Alto, CA 94304.
32. Storms, E., A critical evaluation of the Pons-Fleischmann effect: Part 1. Infinite Energy, 2000. 6(31): p. 10.
33. Gammon, B.E., Cathode cooling by expansion of hydrogen in calorimetric tests for cold fusion. Fusion Technol., 1993. 23: p. 342.
34. Lyakhov, B.F., et al., Anomalous heat release in the Pd/PdO system electrolytically saturated with hydrogen. Russ. J. Phys. Chem., 1993. 67: p. 491.
35. Rittner, E.S. and A. Meulenberg Jr., A chemical interpretation of heat generated in 'cold fusion'. J. Fusion Energy, 1990. 9: p. 377.
36. Cunnane, V.J., R.A. Scannell, and D.J. Schiffrin, H2 + O2 recombination in non-isothermal, non-adiabatic electrochemical calorimetry of water electrolysis in an undivided cell. J. Electroanal. Chem., 1989. 269: p. 163.
37. Joncich, M.J. and N. Hackerman, The Reaction of Hydrogen and Oxygen on Submerged Platinum Electrode Catalysts. I. Effect of Stirring, Temperarture and Electric Polarization. J. Phys. Chem., 1953. 57: p. 674.
38. Will, F., Hydrogen + oxygen recombination and related heat generation in undivided electrolysis cells. J. Electroanal. Chem., 1997. 426: p. 177.
39. Sun, D.-L., et al., An explanation for the abnormal temperature rise of palladium cathode during electrochemical deuterium charging. Science in China A, 1993. 36: p. 1501.
40. Wan, C.M., et al. Repeated Heat Bursts in the Electrolysis of D2O. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
41. Mazzolai, F.M., P.G. Bordoni, and F.A. Lewis, Elastic Energy Dissipation Effects in the Palladium-Hydrogen System. J. Less Common Metals, 1980. 74: p. 137.
42. AbuTaha, A.F., Cold fusion - the heat mechanism. J. Fusion Energy, 1990. 9(3): p. 345.
43. Holst-Hansen, P. and D. Britz, Can current fluctuations account for the excess heat claims of Fleischmann and Pons? J. Electroanal. Chem., 1995. 388: p. 11.
44. Storms, E., Measurements of excess heat from a Pons-Fleischmann-type electrolytic cell using palladium sheet. Fusion Technol., 1993. 23: p. 230.
45. Handel, P.H., Thermoelectric excess heat effect in electrolytic cells. Z. Phys. B, 1994. 95: p. 489.
46. Kainthla, R.C., et al., Eight chemical explanations of the Fleischmann-Pons effect. J. Hydrogen Energy, 1989. 14(11): p. 771.
47. Fleischmann, M., et al., Calorimetry of the palladium-deuterium-heavy water system. J. Electroanal. Chem., 1990. 287: p. 293.
48. Guruswamy, S. and M.E. Wadsworth. Metallurgical Aspects in Cold Fusion Experiments. in The First Annual Conference on Cold Fusion. 1990. University of Utah Research Park, Salt Lake City, Utah: National Cold Fusion Institute.
49. Johnson, K.H., Jahn-Teller Symmetry Breaking and Hydrogen Energy in g-PdD "Cold Fusion". Trans. Fusion Technol., 1994. 26(4T): p. 427.
50. Shanahan, K., A Systematic Error in Mass Flow Calorimetry Demonstrated. Thermochimica Acta, 2001.
51. Storms, E. Excess Power Production from Platinum Cathodes Using the Pons-Fleischmann Effect. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
52. Arata, Y. and Y.-C. Zhang, Anomalous 'deuterium-reaction energies' within solid. Proc. Japan. Acad., 1998. 74 B: p. 155.
53. Arata, Y. and Y.-C. Zhang, Critical condition to induce 'excess energy' within [DS-H2O] cell. Proc. Japan Acad., 1999. 75 Ser. B: p. 76.
54. Arata, Y. and Y.-C. Zhang, Definitive difference between [DS-D2O] and [Bulk-D2O] cells in 'deuterium- reaction'. Proc. Japan Acad., 1999. 75 Ser. B: p. 71.
55. Arata, Y. and Y.-C. Zhang, Anomalous difference between reaction energies generated within D2O-cell and H2O-cell. Jpn. J. Appl. Phys. Pt.2, 1998. 37: p. L1274.
56. McKubre, M.C.H., et al. The Emergence of a Coherent Explanation for Anomalies Observed in D/Pd and H/Pd System: Evidence for 4He and 3He Production. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
57. Clarke, W.B., Search for 3He and 4He in Arata-Style Palladium Cathodes I: A Negative Result. Fusion Sci. and Technol., 2001. 40.
58. Clarke, B.W., et al., Search for 3He and 4He in Arata-Style Palladium Cathodes II: Evidence for Tritium Production. Fusion Sci. and Technol., 2001. 40.
59. Case, L.C. Catalytic Fusion of Deuterium into Helium-4. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
60. Mallove, E., Progress in catalytic fusion. Infinite Energy, 1999. 4(23): p. 9.
61. Storms, E. Ways to Initiate a Nuclear Reaction in Solid Environments. in American Physical Society Meeting. 2001. Seattle, WA.
62. Mizuno, T., et al., Anomalous heat evolution from a solid-state electrolyte under alternating current in high-temperature D2 gas. Fusion Technol., 1996. 29: p. 385.
63. Mizuno, T., et al., Anomalous gamma peak evolution from SrCe solid state electrolyte charged in D2 gas. Int. J. Hydrogen Energy, 1997. 22: p. 23.
64. Mizuno, T., et al., Formation of 197Pt radioisotopes in solid state electrolyte treated by high temperature electrolysis in D2 gas. Infinite Energy, 1995. 1(4): p. 9.
65. Jorné, J., Neutron emission studies during the electrolysis of deuterium by using BaCeO3 solid electrolyte and palladium electrodes. Fusion Technol., 1994. 26: p. 244.
66. Oriani, R.A., An investigation of anomalous thermal power generation from a proton-conducting oxide. Fusion Technol., 1996. 30: p. 281.
67. Samgin, A.L., et al. Cold Fusion and Anomalous Effects in Deuteron Conductors During Non-Stationary High-Temperature Electrolysis. in 5th International Conference on Cold Fusion. 1995. Monte-Carlo, Monaco: IMRA Europe, Sophia Antipolis Cedex, France.
68. Biberian, J.-P., et al. Electrolysis of LaAlO3 Single Crystals and Ceramics in a Deuteriated Atmosphere. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
69. Melich, M.E. and W.N. Hansen. Some Lessons from 3 Years of Electrochemical Calorimetry. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
70. Miles, M.H. and B.F. Bush. Calorimetric Principles and Problems in Pd-D2O Electrolysis. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
71. Miles, M., M.A. Imam, and M. Fleischmann. "Case Studies" of Two Experiments Carried Out With the ICARUS Systems. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
72. Noninski, V.C. and C.I. Noninski, Comments on 'measurement and analysis of neutron and gamma-ray emission rates, other fusion products, and power in electrochemical cells having palladium cathodes'. Fusion Technol., 1991. 19: p. 579.
73. Noninski, V.C. and C.I. Noninski, Notes on two papers claiming no evidence for the existence of excess energy during the electrolysis of 0.1M LiOD/D2O with palladium cathodes. Fusion Technol., 1993. 23: p. 474.
74. De Ninno, A., A. La Barbera, and V. Violant, Deformations induced by high loading ratios in palladium-deuterium compounds. J. Alloys and Compounds, 1997. 253-254: p. 181.
75. Lynch, J.F., J.D. Clewley, and T.B. Flanagan, The Formation of Voids in Palladium Metal by the Introduction and Removal of Interstital Hydrogen. Phil. Mag., 1973. 28: p. 1415.
76. Miles, M.H. and K.B. Johnson, Anomalous Effects in Deuterated Systems, Final Report. 1996.
77. Kobayashi, M., et al. Measurements of D/Pd and Excess Heat during Electrolysis of LiOD in a Fuel-Cell Type Closed Cell Using a Palladium Sheet Cathode. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
78. Miyamaru, H. and A. Takahashi. Periodically Current-Controlled Electrolysis of D2O/Pd System for Excess Heat Production. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
79. Huang, N. Effect of Light Water Additions on Excess Heat Generation of Palladium Deuterium System. in 8th World Hydrogen Energy Conf. 1990. Honolulu, HI: Hawaii Natural Energy Insitute, 2540 Dole St., Holmes Hall 246, Honolulu, HI 96822.
80. Belzner, A., et al., Two fast mixed-conductor systems: deuterium and hydrogen in palladium - thermal measurements and experimental considerations. J. Fusion Energy, 1990. 9(2): p. 219.
81. McKubre, M.C.H., et al. New Hydrogen Energy Research at SRI. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
82. Storms, E. and C. Talcott-Storms, The effect of hydriding on the physical structure of palladium and on the release of contained tritium. Fusion Technol., 1991. 20: p. 246.
83. Miles, M.H., M. Fleischmann, and M.A. Imam, Calorimetric Analysis of a Heavy Water Electrolysis Experiment Using a Pd-B Alloy Cathode. 2001, Naval Research Laboratory, NRL/MR/6320--01-8526: Washington. p. 154.
84. Szpak, S., P.A. Mosier-Boss, and M.H. Miles, Calorimetry of the Pd+D codeposition. Fusion Technol., 1999. 36: p. 234.
85. Iwamura, Y., et al. Detection of Anomalous Elements, X-ray and Excess Heat Induced by Continous Diffusion of Deuterium Through Multi-layer Cathode (Pd/CaO/Pd). in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
86. Klopfenstein, M.F. and J. Dash. Thermal Imaging during Electrolysis of Heavy Water with a Ti Cathode. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: Vancouver, Canada.
87. Warner, J. and J. Dash. Heat Produced During the Electrolysis of D2O with Titanium Cathodes. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
88. Bernardini, M., et al. Anomalous Effects Induced by D2O Electrolysis at Titanium. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
89. Miao, B., Experimental exploration on the possible mechanism of D-D cold fusion in titanium lattice. Xibei Shifan Xuebao. Ziran Kexueban, 1994. 30(1): p. 39 (in Chinese).
90. Zhang, Q., et al., The excess heat experiments on cold fusion in titanium lattice. Chin. J. Atom. Mol. Phys., 1995. 12(2): p. 165.
91. Klyuev, V.A., et al., High-energy Processes Accompanying the Fracture of Solids. Sov. Tech. Phys. Lett., 1986. 12: p. 551.
92. Dickinson, J.T., et al., Fracto-emission from deuterated titanium: Supporting evidence for a fracto-fusion mechanism. J. Mater. Res., 1990. 5: p. 109.
93. Preparata, G., A new look at solid-state fractures, particle emission and 'cold' nuclear fusion. Nuovo Cimento A, 1991. 104: p. 1259.
94. Fateev, E.G., Possibilities for establishing the mechanism of neutron generation in deuterated materials under mechanical loading. Tech. Phys. Lett., 1995. 21(5): p. 373.
95. Storms, E.K., A Study of Those Properties of Palladium That Influence Excess Energy Production by the "Pons-Fleischmann" Effect. Infinite Energy, 1996. 2(8): p. 50.
96. Mizuno, T., et al., Neutron Evolution from a Palladium Electrode by Alternate Absorption Treatment of Deuterium and Hydrogen. Jpn. J. Appl. Phys., 2001. 40: p. L989.
97. Chien, C.-C., et al., On an electrode producing massive quantities of tritium and helium. J. Electroanal. Chem., 1992. 338: p. 189.
98. Taubes, G., Cold Fusion Conundrum at Texas A & M. Science, 1990. 248: p. 1299.
99. Cedzynska, K., et al., Tritium analysis in palladium with an open system analytical procedure. Fusion Technol., 1991. 20: p. 108.
100. Cedzynska, K. and F.G. Will, Closed-system analysis of tritium in palladium. Fusion Technol., 1992. 22: p. 156.
101. Boucher, G.R., F.E. Collins, and R.L. Matlock, Separation factors for hydrogen isotopes on palladium. Fusion Technol., 1993. 24: p. 200.
102. Corrigan, D.A. and E.W. Schneider, Tritium separation effects during heavy water electrolysis: implications for reported observations of cold fusion. J. Electroanal. Chem., 1990. 281: p. 305.
103. Storms, E. and C. Talcott, Electrolytic tritium production. Fusion Technol., 1990. 17: p. 680.
104. Claytor, T.N., D.D. Jackson, and D.G. Tuggle, Tritium production from low voltage deuterium discharge on palladium and other metals. Infinite Energy, 1996. 2(7): p. 19.
105. Claytor, T.N., et al. Tritium Production from Palladium Alloys. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
106. Arata, Y. and Y.-C. Zhang, Helium (4He, 3He) within deuterated Pd-black. Proc. Japan Acad. B, 1997. 73: p. 1.
107. Abell, G.C., et al., Helium release from aged palladium tritide. Phys. Rev. B: Condens. Matter, 1990. 41(2): p. 1220.
108. Miles, M.H., et al., Correlation of excess power and helium production during D2O and H2O electrolysis using palladium cathodes. J. Electroanal. Chem., 1993. 346: p. 99.
109. Miles, M.H., B.F. Bush, and J.J. Lagowski, Anomalous effects involving excess power, radiation, and helium production during D2O electrolysis using palladium cathodes. Fusion Technol., 1994. 25: p. 478.
110. Miles, M.H. and B.F. Bush, Heat and Helium Measurements in Deuterated Palladium. Trans. Fusion Technol., 1994. 26(4T): p. 156.
111. Bush, B.F., et al., Helium production during the electrolysis of D2O in cold fusion experiments. J. Electroanal. Chem., 1991. 304: p. 271.
112. Bush, B. and J.J. Lagowski. Methods of Generating Excess Heat with the Pons and Fleischmann Effect: Rigorous and Cost Effective Calorimetry, Nuclear Products Analysis of the Cathode and Helium Analysis. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
113. Jones, S.E. and L.D. Hansen, Examination of claims of Miles et al in Pons-Fleischmann-Type cold fusion experiments. J. Phys. Chem., 1995. 99: p. 6966.
114. Miles, M.H. and C.P. Jones, Cold fusion experimenter Miles responds to critic. 21st Century Science & Technology, 1992. Spring: p. 75.
115. Miles, M.H., Reply to 'Examination of claims of Miles et al. in Pons-Fleischmann-type cold fusion experiments'. J. Phys. Chem. B, 1998. 102: p. 3642.
116. Jones, S.E., L.D. Hansen, and D.S. Shelton, An assessment of claims of excess heat in cold fusion calorimetry. J. Phys. Chem. B, 1998. 102: p. 3647.
117. Gozzi, D., et al., Quantitative measurements of helium-4 in the gas phase of Pd + D2O electrolysis. J. Electroanal.Chem., 1995. 380: p. 109.
118. Gozzi, D., et al., X-ray, heat excess and 4He in the D/Pd system. J. Electroanal. Chem., 1998. 452: p. 251.
119. Chubb, S.R. and T.A. Chubb. Quantum Mechanics of "Cold and "Not-So-Cold" Fusion". in The First Annual Conference on Cold Fusion. 1990. University of Utah Research Park, Salt Lake City, Utah: National Cold Fusion Institute.
120. Takahashi, A., Some Considerations of Multibody Fusion in Metal-Deuterides. Trans. Fusion Technol., 1994. 26(4T): p. 451.
121. Takahashi, A., et al., Detection of three-body deuteron fusion in titanium deuteride under the stimulation by a deuteron beam. Phys. Lett. A, 1999. 255: p. 89.
122. Hanawa, T. X-ray Spectroscropic Analysis of Carbon Arc Products in Water. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
123. Sundaresan, R. and J.O.M. Bockris, Anomalous Reactions During Arcing Between Carbon Rods in Water. Fusion Technol., 1994. 26: p. 261.
124. Ransford, H.E., Non-Stellar nucleosynthesis: Transition metal production by DC plasma-discharge electrolysis using carbon electrodes in a non-metallic cell. Infinite Energy, 1999. 4(23): p. 16.
125. Mizuno, T., et al. Confirmation of Heat Generation and Anomalous Element Caused by Plasma Electrolysis in the Liquid. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
126. Ohmori, T. and T. Mizuno, Nuclear transmutation reaction caused by light water electrolysis on tungsten cathode under incandescent conditions. Infinite Energy, 1999. 5(27): p. 34.
127. Ohmori, T. and T. Mizuno. Strong Excess Energy Evolution, New Element Production, and Electromagnetic Wave and/or Neutron Emission in the Light Water Electrolysis with a Tungsten Cathode. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
128. Celani, F., et al. Preliminary Results with "Cincinnati Group Cell" on Thorium "Transmutation" under 50 Hz AC Excitation. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
129. Fox, H. and S.-X. Jin, Operating the LENT-1 transmutation reactor: Preliminary report. J. New Energy, 1997. 2(2): p. 110.
130. Miley, G. On the Reaction Product and Heat Correlation for LENRs. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
131. Miley, G.H. and J.A. Patterson, Nuclear transmutations in thin-film nickel coatings undergoing electrolysis. J. New Energy, 1996. 1(3): p. 5.
132. Hora, H., J.C. Kelly, and G. Miley, Energy gain and nuclear transmutation by low-energy p- or d-reaction in metal lattices. Infinite Energy, 1997. 2(12): p. 48.
133. Komaki, H., production de proteins par 29 souches de microorganismes et augmentation du potassium en milieu de culture sodique sans potassium. Revue de Pathologie Comparee, 1967. 67: p. 213.
134. Komaki, H., Formation de protines et variations minerales par des microorganismes en milieu de culture, sort avec or sans potassium, sort avec ou sans phosphore. Revue de Pathologie Comparee, 1969. 69: p. 83.
135. Komaki, H. Observations on the Biological Cold Fusion or the Biological Transformation of Elements. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
136. Komaki, H. An Approach to the Probable Mechanism of the Non-Radioactive Biological Cold Fusion or So-Called Kervran Effect (Part 2). in Fourth International Conference on Cold Fusion. 1993. Lahaina, Maui: Electric Power Research Institute 3412 Hillview Ave., Palo Alto, CA 94304.
137. Komaki, H. and C.L. Kervran. Experiences de Komaki, Premiere Serie de Recherches. in Preuves en biologie de transmutations a faible energie. 1975. maloine, S. A. , Paris.
138. Thompkins, P. and C. Byrd, The Secret Life of Plants. 1993, New York: Penguin Books.
139. Kervran, C.L., Biological Transmutations. 1972: Swan House Publishing Co.
140. Vysotskii, V.I., A.A. Kornilova, and I.I. Samoyloylenko. Experimental discovery of phenomenon of low-energy nuclear transformation of isotopes (Mn55=Fe57) in growing biological cultures. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
141. Vysotskii, V., et al., Observation and mass-spectrometry. Study of controlled transmutation of intermediate mass isotopes in growing biological cultures. Infinite Energy, 2001. 6(36): p. 64.
142. Jiang, X.-L., et al. Tip Effect and Nuclear-Active Sites. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
143. Dash, J., G. Noble, and D. Diman, Surface Morphology and Microcomposition of Palladium Cathodes After Electrolysis in Acified Light and Heavy Water: Correlation With Excess Heat. Trans. Fusion Technol., 1994. 26(4T): p. 299.
144. Silver, D.S., J. Dash, and P.S. Keefe, Surface topography of a palladium cathode after electrolysis in heavy water. Fusion Technol., 1993. 24: p. 423.
145. Storms, E.K. Some Thoughts on the Nature of the Nuclear-Active Regions in Palladium. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
146. Oya, Y., et al. Material Conditions to Replicate the Generation of Excess Energy and the Emission of Excess Neutrons. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
147. Storms, E. Relationship Between Open-Circuit-Voltage and Heat Production in a Pons-Fleischmann Cell. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
148. Celani, F., et al. D/Pd loading ratio up to 1.2: by high power µS pulsed electrolysis in Pd plates. in Cold Fusion and Advanced Energy Sources. 1994. Belarusian State University, Minsk, Belarus.
149. Czerwinski, A., Influence of lithium cations on hydrogen and deuterium electrosorption in palladium. Electrochim. Acta, 1994. 39: p. 431.
150. Asami, N., et al. Material Behavior of Highly Deuterium Loaded Palladium by Electrolysis. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
151. Oya, Y., et al. A Role of Alkaline Ions for Dynamic Movement of Hydrogen Isotopes in Pd. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
152. Yamazaki, O., et al., Hydrogen absorption and Li inclusion in a Pd cathode in LiOH solution. J. Electroanal. Chem., 1995. 390: p. 127.
153. Mebrahtu, T., et al., Observations on the surface composition of palladium cathodes after D2O electrolysis in LiOD solutions. J. Electroanal. Chem., 1989. 267: p. 351.
154. Ulman, M., et al., Surface and electrochemical characterization of Pd cathodes after prolonged charging in LiOD + D2O solutions. J. Electroanal. Chem., 1990. 286: p. 257.
155. Brillas, E., et al., Product analysis from D2O electrolysis with Pd and Ti cathodes. Electrochim. Acta, 1992. 37(2): p. 215.
156. Iwamura, Y., et al., Detection of anomalous elements, X-ray and excess heat induced by continuous diffusion of deuterium through multi-layer cathode (Pd/CaO/Pd). Infinite Energy, 1998. 4(20): p. 56.
157. Iwamura, Y., et al., Detection of anomalous elements, x-ray, and excess heat in a D2-Pd system and its interpretation by the electron-induced nuclear reaction model. Fusion Technol., 1998. 33: p. 476.
158. McKubre, M.C.H., et al. Concerning Reproducibility of Excess Power Production. in 5th International Conference on Cold Fusion. 1995. Monte-Carlo, Monaco: IMRA Europe, Sophia Antipolis Cedex, France.
159. Chechin, V.A., et al., Critical review of theoretical models for anomalous effects in deuterated metals. Int. J. Theo. Phys., 1994. 33: p. 617.
160. Preparata, G., Some theories of 'cold' nuclear fusion: a review. Fusion Technol., 1991. 20: p. 82.
161. Fleischmann, M., S. Pons, and G. Preparata, Possible theories of cold fusion. Nuovo Cimento A, 1994. 107: p. 143.
162. Kasagi, J., et al. Anomalously Enhanced D(d,p)T Reaction in Pd and PdO Observed at Very Low Bombarding Energies. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
163. Kasagi, J., et al. Low Energy Nuclear Fusion Reactions in Solids. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
164. Kim, Y.E., Cross section for cold deuterium-deuterium fusion. Fusion Technol., 1990. 17: p. 507.
165. Beuhler, R.J., G. Friedlander, and L. Friedman, Cluster-Impact Fusion. Phys. Rev. Lett., 1990. 63: p. 1292.
166. Beuhler, R.J., G. Friedlander, and L. Friedman, Cluster-impact Fusion [Erratum]. Phys. Rev. Lett., 1992. 88: p. 2108.
167. Kozima, H., K. Kaki, and M. Ohta, Anomalous phenomenon in solids described by the TCNF model. Fusion Technol., 1998. 33: p. 52.
168. Mayer, F.J. and J.R. Reitz, Nuclear energy release in metals. Fusion Technol., 1991. 19: p. 552.
169. Russell Jr., J.L., Plausibility argument for a suggested mechanism for cold fusion. Ann. Nucl. Energy, 1990. 17(10): p. 545.
170. Yang, J., L. Tang, and X. Chen, Dineutron model research of cold fusion. Acta Sci. Nat. Univ. Norm. Hunan, 1996. 19(2): p. 25.
171. Swartz, M., Possible deuterium production from light water excess enthalpy experiments using nickel cathodes. J. New Energy, 1996. 1(3): p. 68.
172. Pokropivnyi, V.V., Bineutron theory of cold nuclear fusion. Dokl. Akad. Nauk. Ukr., 1993(4): p. 86 (in Russian).
173. Timashev, S.F., Possible mechanisms for nuclear-chemical transformations in a palladium matrix during heavy water electrolysis. Zh. Fiz. Khim, 1989. 63: p. 2283 (in Russian).
174. Cerofolini, G.F. and A.F. Para, Can binuclear atoms solve the cold fusion puzzle? Fusion Technol., 1993. 23: p. 98.
175. Conte, E., Theoretical indications of the possibility of nuclear reactions at low energy. Infinite Energy, 1999. 4(24): p. 49.
176. Phipps Jr., T.E., Neutron formation by electron penetration of the nucleus. Infinite Energy, 1999. 5(26): p. 58.
177. Schultz, R. and J.P. Kenny, Electronuclear catalysts and initiators: The di-neutron model for cold fusion. Infinite Energy, 1999. 5(29): p. 58.
178. Andermann, G. A Theoretical Model (Nu-Q) for Rationalizing Electrochemically Induced Nuclear Events Observed in Deuterium Loaded Pd Cathodes. in The First Annual Conference on Cold Fusion. 1990. University of Utah Research Park, Salt Lake City, Utah: National Cold Fusion Institute.
179. Moon, D., Addendum to "Mechanisms of a disobedient science". Infinite Energy, 1996. 1(5/6): p. 89.
180. Lipson, A.G. and D.M. Sakov, Increase in the intensity of the external neutron flux in the irradiation of a KD2PO4 crystal at the point of the ferroelectric transition. Tech. Phys. Lett., 1994. 20: p. 954.
181. Roussetski, A.S. Investigation of Nuclear Emissions in the Process of D(H) Escaping from Deuterized (Hydrogenized) PdO-Pd-PdO and PdO-Ag Samples. in Sixth International Conference on Cold Fusion,Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
182. Stella, B., et al. Evidence for Stimulated Emission of Neutrons in Deuterated Palladium. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
183. Lipson, A.G. and D.M. Sakov. Amplification of the Neutron Flux Transmitted Through KD2PO4 Single-Crystal at the Ferroelectric Phase Transition State. in 5th International Conference on Cold Fusion. 1995. Monte-Carlo, Monaco: IMRA Europe, Sophia Antipolis Cedex, France.
184. Oya, Y., et al. Hydrogen Isotope Effect Induced by Neutron Irradiation in Pd-LiOD(H) Electrolysis. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
185. Fisher, J.C., Polyneutrons as agents for cold nuclear reactions. Fusion Technol., 1992. 22: p. 511.
186. Fisher, J.C., Liquid-drop model for extremely neutron rich nuclei. Fusion Technol., 1998. 34: p. 66.
187. Oriani, R.A., Anomalous heavy atomic masses produced by electrolysis. Fusion Technol., 1998. 34: p. 76.
188. Chubb, S.R. and T.A. Chubb, Ion band state fusion: reactions, power density, and the quantum reality question. Fusion Technol., 1993. 24: p. 403.
189. Chubb, T.A. and S.R. Chubb, Cold fusion as an interaction between ion band states. Fusion Technol., 1991. 20: p. 93.
190. Chubb, T.A. and S.R. Chubb. Deuteron Fluxing and the Ion Band State Theory. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
191. Liboff, R.L., Fusion via metallic deuterium. Phys. Lett., 1979. 71A: p. 361.
192. Liboff, R.L., Feasibility of fusion of an aggregate of deuterons in the ground state. Phys.Lett., 1993. 174 A: p. 317.
193. Hagelstein, P.L. A Unified Model for Anomalies in Metal Deuterides. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
194. Kucherov, Y. Slow Nuclear Excitation Model. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
195. Turner, L., Thoughts Unbottled by Cold Fusion. Phys. Today, 1989. Sept.: p. 140.
196. Kim, Y.E. and A.L. Zubarev, Gamow factor cancellation and nuclear physics mechanisms for anomalous low-energy nuclear reactions. J. New Energy, 1996. 1(3): p. 145.
197. Kim, Y.E., et al., Reaction Barrier Transparency for Cold Fusion with Deuterium and Hydrogen. Trans. Fusion Technol., 1994. 26(4T): p. 408.
198. Li, X.Z. Tunneling the Coulomb barrier via lattice confined ions. in 5th International Conference on Cold Fusion. 1995. Monte-Carlo, Monaco: IMRA Europe, Sophia Antipolis Cedex, France.
199. Jones, S.E., et al., Observation of cold nuclear fusion in condensed matter. Nature, 1989. 338: p. 737.
200. Parmenter, R.H., A possible scenario for the onset of cold fusion in deuterated metals. Infinite Energy, 1998. 4(21): p. 41.
201. Preparata, G. Cold Fusion '93': Some Theoretical Ideas. in Fourth International Conference on Cold Fusion. 1993. Lahaina, Maui: Electric Power Research Institute 3412 Hillview Ave., Palo Alto, CA 94304.
202. Flanagan, T.B. and W.A. Oates, The Palladium-Hydrogen System. Annu. Rev. Mater. Sci., 1991. 21: p. 269.
203. Worsham Jr., J.E., M.K. Wilkinson, and C.G. Shull, Neutron-Diffraction Observations on the Palladium-hydrogen and Palladium-deuterium systems. J. Phys. Chem. Solids, 1957. 3: p. 303.
204. Dillon, C.T., B.J. Kennedy, and M.M. Elcombe, The electrochemically formed palladium-deuterium system. II. In situ neutron diffraction studies. Aust. J. Chem., 1993. 46: p. 681.
205. Batalla, E., E.G. Zwartz, and B.A. Judd, In-situ X-ray diffraction of palladium cathodes in electrolytic cells. Solid State Commun., 1989. 71: p. 805.
206. Anderson, I.S., D.K. Ross, and C.J. Carlile, The Structure of the g Phase of Palladium Deuteride. Phys. Lett. A, 1978. 68: p. 249.
207. Lo, S.Y., Enhancement of nuclear fusion in a strongly coupled cold plasma. Mod. Phys. Lett. B, 1989. 3(16): p. 1207.
208. Bazhutov, Y.N., B.A. Khrenov, and G.B. Khristiansen, About one opportunity of second shower spectrum interpretation observed at small depth underground. Isv. AN USSR, ser. phys., 1982. 46(9): p. 2425.
209. Bazhutov, Y.N., et al. Interpretation of cold nuclear fusion by means of erzion catalysis. in Fiz. Plazmy Nekotor. Vopr. Obshch. Fiz. M. 1990.
210. Bazhutov, Y.N. Erzion Discovery in Cosmic Rays and its Possible Great Role in Nature in Framework of Erzion Model of Cold Nuclear Transmutation. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
211. Rafelski, J., et al., Nuclear reactions catalyzed by a massive negatively charged particle. How Cold Fusion Can Be Catalyzed. Fusion Technol., 1990. 18: p. 136.
212. McKibben, J.L., Recent observations that yield information on catalytic particles. Infinite Energy, 1998. 4(20): p. 70.
213. McKibben, J.L., Can Cold Fusion be Catalyzed by Fractionally-Charged Ions that have Evaded FC Particle Searches. Infinite Energy, 1995. 1(4): p. 14.
214. Dufour, J., Cold fusion by sparking in hydrogen isotopes. Fusion Technol., 1993. 24: p. 205.
215. Dufour, J., Energy Source System: World Patent, WO 91/01036 (1991).
216. Dufour, J., et al. The Hydrex Concept-Effect on Heavy Nuclei. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
217. Dufour, J., et al. Hydrex Catallyzed Transmutation of Uranium and Palladium: Experimental Part. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
218. Matsumoto, T., 'Nattoh' model for cold fusion. Fusion Technol., 1989. 16: p. 532.
219. Matsumoto, T., Observation of meshlike traces on nuclear emulsions during cold fusion. Fusion Technol., 1993. 23: p. 103.
220. Matsumoto, T., Observation of gravity decays of multiple-neutron nuclei during cold fusion. Fusion Technol., 1992. 22: p. 164.
221. Shoulders, K. and S. Shoulders, Observations on the role of charge clusters in nuclear cluster reactions. J. New Energy, 1996. 1(3): p. 111.
222. Shoulders, K.R.: US Patent 5,018,180 (1991); 5,054,046 (1991); 5,054,047 (1991); 5,123,039 (1992) and 5,148,461 (1992).
223. Fox, H., Charge clusters in operation. Infinite Energy, 1997. 2(12): p. 62.
224. Fox, H. and J.S. X., Low-energy nuclear reactions and high-density charge clusters. Infinite Energy, 1998. 4(20): p. 26.
225. Bhadkamkar, A. and H. Fox, Electron Charge Cluster Sparking in Aqueous Solutions. J. New Energy, 1996. 1(4): p. 62.
226. Lewis, E., Comments on 'Transmutation in a gold-light water electrolysis system'. Fusion Technol., 1999. 36: p. 242.
227. Park, R., Voodoo Science. 2000, New York, NY: Oxford University Press. 211 pages.
228. Bockris, J.O.M., Accountibility and academic freedom: The battle concerning research on cold fusion at Texas A&M University. Accountibility in Research, 2000. 8: p. 103.
229. Mallove, E.F., The triumph of alchemy: Professor John Bockris and the transmutation crisis at Texas A&M. Infinite Energy, 2000. 6(32): p. 9.
230. Miley, G.H., Some personal reflections on scientific ethics and the cold fusion 'episode'. Accountability Res., 2000. 8: p. 121.
231. Beaudette, C.G., Excess Heat. Why Cold Fusion Research Prevailed. 2000, Concord, NH: Oak Grove Press (Infinite Energy, Distributor). 365 pages.
232. Mallove, E. and J. Rothwell, The pseudoscientists of APS. Infinite Energy, 1999. 5(25): p. 23.
233. Riley, D. and M. McLaughlin, Turning thre corner: Energy solutions for the 21st century. Vol. 1. 2001, Tahoe City, CA: Alternative Energy Institute, Inc. 385.
References for Table 2
1. Huang, N., et al. A Flow Calorimeter Used in Duplication of 'Cold Fusion'. in Special Session Cold Fusion, Electrochemical Society. 1989. Hollywood, Fl: Electrochemical Society.
2. Kainthla, R.C., et al., Sporadic observation of the Fleischmann-Pons heat effect. Electrochim. Acta, 1989. 34: p. 1315.
3. Santhanam, K.S.V., et al., Electrochemically initiated cold fusion of deuterium. Indian J. Technol., 1989. 27: p. 175.
4. Appleby, A.J., et al. Anomalous Calorimetric Results During Long-Term Evolution of Deuterium on Palladium from Alkaline Deuteroxide Electrolyte. in The First Annual Conference on Cold Fusion. 1990. University of Utah Research Park, Salt Lake City, Utah: National Cold Fusion Institute.
5. Belzner, A., et al., Two fast mixed-conductor systems: deuterium and hydrogen in palladium - thermal measurements and experimental considerations. J. Fusion Energy, 1990. 9(2): p. 219.
6. Eagleton, R.D. and R.T. Bush, Calorimetric experiments supporting the transmission resonance model for cold fusion. Fusion Technol., 1991. 20: p. 239.
7. Scott, C.D., et al., Measurement of excess heat and apparent coincident increases in the neutron and gamma-ray count rates during the electrolysis of heavy water. Fusion Technol., 1990. 18: p. 103.
8. Fleischmann, M., et al., Calorimetry of the palladium-deuterium-heavy water system. J. Electroanal. Chem., 1990. 287: p. 293.
9. Hutchinson, D.P., et al., Initial Calorimetry Experiments in the Physics Division -ORNL. 1990, Oak Ridge National Laboratory ORNL/TM-11356: Oak Ridge, TN.
10. Zahm, L.L., et al., Experimental investigations of the electrolysis of D2O using palladium cathodes and platinum anodes. J. Electroanal. Chem., 1990. 281: p. 313.
11. Miles, M.H., K.H. Park, and D.E. Stilwell, Electrochemical calorimetric evidence for cold fusion in the palladium-deuterium system. J. Electroanal. Chem., 1990. 296: p. 241.
12. Oriani, R.A., et al., Calorimetric measurements of excess power output during the cathodic charging of deuterium into palladium. Fusion Technol., 1990. 18: p. 652.
13. Yang, C.-S., et al. Observation of Excess Heat and Tritium on Electrolysis of D2O. in 8th World Hydrogen Energy Conf. 1990. Honolulu, HI: Hawaii Natural Energy Insitute, 2540 Dole St., Holmes Hall 246, Honolulu, HI 96822.
14. Zhang, Z.L., et al. Calorimetric Observation Combined with the Detection of Particle Emissions During the Electrolysis of Heavy Water. in Anomalous Nuclear Effects in Deuterium/Solid Systems, "AIP Conference Proceedings 228". 1990. Brigham Young Univ., Provo, UT: American Institute of Physics, New York.
15. Bertalot, L., et al. Analysis of Tritium and Heat Excess in Electrochemical Cells With Pd Cathodes. in Second Annual Conference on Cold Fusion, "The Science of Cold Fusion". 1991. Como, Italy: Societa Italiana di Fisica, Bologna, Italy.
16. Bush, B.F., et al., Helium production during the electrolysis of D2O in cold fusion experiments. J. Electroanal. Chem., 1991. 304: p. 271.
17. McKubre, M.C.H., et al. Isothermal Flow Calorimetric Investigations of the D/Pd System. in Second Annual Conference on Cold Fusion, "The Science of Cold Fusion". 1991. Como, Italy: Societa Italiana di Fisica, Bologna, Italy.
18. Noninski, V.C. and C.I. Noninski, Determination of the excess energy obtained during the electrolysis of heavy water. Fusion Technol., 1991. 19: p. 364.
19. Yun, K.-S., et al., Calorimetric observation of heat production during electrolysis of 0.1 M LiOD + D2O solution. J. Electroanal. Chem., 1991. 306: p. 279.
20. Bertalot, L., et al. Study of Deuterium Charging in Palladium by the Electrolysis of Heavy Water: Search for Heat Excess and Nuclear Ashes. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
21. Gozzi, D., et al. Experiments with Global Detection of Cold Fusion Byproducts. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
22. Hasegawa, N., et al. Observation of Excess Heat during Electrolysis of 1 M LiOD in a Fuel Cell Type Closed Cell. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
23. McKubre, M.C.H., et al. Excess Power Observations in Electrochemical Studies of the D/Pd System; The Influence of Loading. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
24. Ota, K., et al. Heat Production at the Heavy Water Electrolysis Using Mechanically Treated Cathode. in Third International Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal Academy Press, Inc., Tokyo, Japan.
25. Storms, E., Measurements of excess heat from a Pons-Fleischmann-type electrolytic cell using palladium sheet. Fusion Technol., 1993. 23: p. 230.
26. Okamoto, M., et al., Excess Heat Generation, Voltage Deviation, and Neutron Emission in D2O-LiOD Systems. Trans. Fusion Technol., 1994. 26(4T): p. 176.
27. Storms, E., Some Characteristics of Heat Production Using the "Cold Fusion" Effect. Trans. Fusion Technol., 1994. 26(4T): p. 96.
28. Bertalot, L., et al. Power Excess Production in Electrolysis Experiments at ENEA Frascati. in 5th International Conference on Cold Fusion. 1995. Monte-Carlo, Monaco: IMRA Europe, Sophia Antipolis Cedex, France.
29. Takahashi, A., et al. Experimental Correlation Between Excess Heat and Nuclear Products. in 5th International Conference on Cold Fusion. 1995. Monte-Carlo, Monaco: IMRA Europe, Sophia Antipolis Cedex, France.
30. Kamimura, H., et al. Excess Heat in Fuel Cell Type Cells from Pure Pd Cathodes Annealed at High Temperatures. in Sixth International Conference on Cold Fusion,Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
31. Yasuda, K., Y. Nitta, and A. Takahashi. Study of Excess Heat and Nuclear Products with Closed D2O Electrolysis Systems. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
32. Ota, K., et al. Heat Measurement During the Heavy Water Electrolysis using Pd Cathode. in The Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
33. Szpak, S., P.A. Mosier-Boss, and M.H. Miles, Calorimetry of the Pd+D codeposition. Fusion Technol., 1999. 36: p. 234.
34. Storms, E. Excess Power Production from Platinum Cathodes Using the Pons-Fleischmann Effect. in 8th International Conference on Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.